Also see:
- Antibody Dependent Enhancement (ADE)
- Dis-information
- Emergency Use Authorization
- Hospital Protocol
- Immune Escape
- Informed Consent
- Isolated Virus
- Mass Vaccinations
- Medical Tyranny
- Natural Immunity
- PCR Testing
- ⇐ back to Problems
- Propaganda
- Statistics
- Vaccine Hesitancy
- Vaccine Injury
- Vaccine Deaths
- Vaccine Monitoring Systems
- Vaccine Performance
- Vaccine Safety
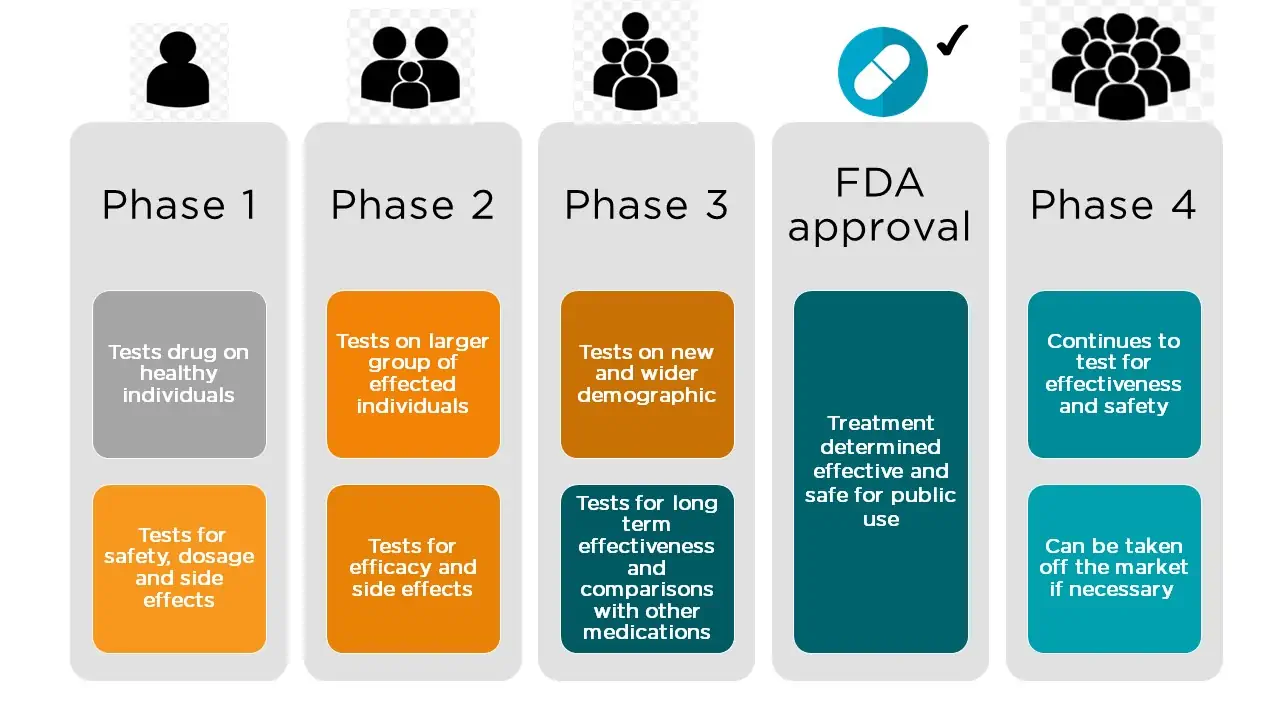
In the classical drug development paradigm, pre-market clinical trials for drugs are conducted in three phases. The trials at each phase have a different purpose and help scientists answer different questions.
- Phase 1 Trials. In phase 1, researchers test the potential product in humans for the first time, to identify rudimentary product characteristics, such as how the body metabolizes a drug and how long it stays in the body, and to provide evidence that the product is not too toxic for further human testing. The treatment group is small (typically 20 – 80 healthy volunteers), but allows researchers to begin to evaluate the treatment’s safety, adjust dosing schemes, and start to identify side effects. This information guides the design of phase 2 studies.
- Phase 2 Trials. Phase 2 studies are intended to explore the effectiveness of the product for a particular indication over a range of doses, and to assess short-term side effects. These studies typically involve a few hundred patients who have the target condition, but do not generally have other diseases that might obscure the effect of the drug on the target condition. Phase 2 trials may be randomized and/or controlled, but often measure laboratory values or other biomarkers rather than clinical outcomes (i.e., effects on how a patient feels, functions, or survives). When a phase 2 study does assess clinical outcomes, it is usually for relatively short periods of time and in a
relatively small number of people. Sponsors assess phase 2 results to determine if the preliminary results are sufficiently promising to justify a phase 3 study. - Phase 3 Trials. Compared to phase 2 trials, the goal of phase 3 trials is to test the experimental product in larger groups of people (typically 300 – 3000), in people who are more similar to those likely to use the product once marketed, and for longer periods of time. Phase 3 studies generally assess clinical outcomes, and are designed to determine whether the demonstrated benefits of the product outweigh its risks.
Source: FDA
Cherry Picking Test Subjects
One method of rigging a trial is to be highly selective of your test subjects. A proper test would include tests on people with various conditions so that the general population could be adequately reflected in the study. The selection and exclusion criteria for COVID-19 clinical trials cherry picked only the healthiest specimens, which does not even remotely exist in the real world.
Clinical Trial Inclusion/Exclusion Criteria Example
source: https://clinicaltrials.gov/ct2/show/NCT04497298?recrs=e&type=Intr&cond=COVID&draw=5&rank=113
| Ages Eligible for Study: | 18 Years to 55 Years (Adult) |
| Sexes Eligible for Study: | All |
| Accepts Healthy Volunteers: | Yes |
Inclusion Criteria:
- Males and females between the ages of 18 and 55 years (at the time of consent). NOTE: Elderly, teenagers, babies excluded, yet governments are saying it is safe for everyone.
- Healthy participant, according to the investigator’s clinical judgment, as established by medical history, vital signs, physical examination, and laboratory assessments NOTE: what stops an investigator from choosing only super-humans?
- Participant with a body mass index (BMI) <30.0 kg/m2 The vast majority of Americans do not fit this criteria.
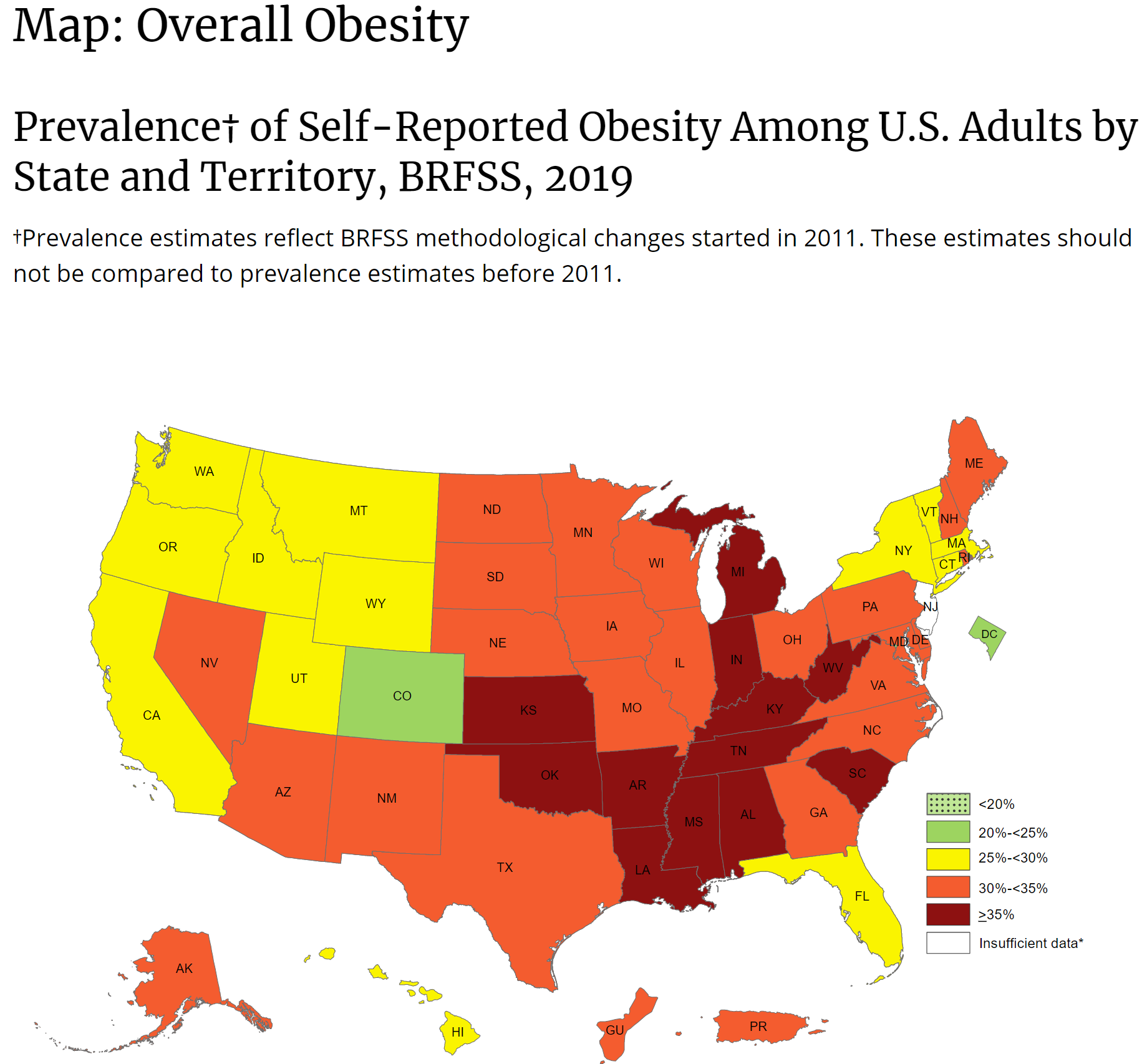
Adult Body Mass Index
source: https://www.cdc.gov/obesity/adult/defining.html
BMI is a person’s weight in kilograms divided by the square of height in meters. A high BMI can indicate high body fatness.
To calculate BMI, see the Adult BMI Calculator or determine BMI by finding your height and weight in this BMI Index Chartexternal icon.
- If your BMI is less than 18.5, it falls within the underweight range.
- If your BMI is 18.5 to <25, it falls within the healthy weight range.
- If your BMI is 25.0 to <30, it falls within the overweight range.
- If your BMI is 30.0 or higher, it falls within the obesity range.
Obesity is frequently subdivided into categories:
- Class 1: BMI of 30 to < 35
- Class 2: BMI of 35 to < 40
- Class 3: BMI of 40 or higher. Class 3 obesity is sometimes categorized as “severe” obesity.
This is a map of obesity (not BMI). Every state has obesity and most states have a population is is highly or mostly obese. If the vaccine was ‘tested’ with non-obese people, what will happen to obese people, who make up the majority of the country?
- Provide written informed consent before initiation of any study procedures. NOTE: What were the test subjects told? Were they told about the spike protein, dangers of Antibody Dependent Enhancement? Once they signed the informed consent declaration, they affirm they were given informed consent. It’s doubtful these people understood the legal ramifications of signing that document.
- A female participant is eligible for this study if she is not pregnant , given by a negative serum pregnancy test at screening and a negative urine pregnancy test at V1 (1st injection), or breast feeding and 1 of the following: NOTE: They screened out every woman who was pregnant or could get pregnant during the trial. But, are these women being followed after the ‘trial’? What has happened to these women after they get pregnant? What are the 30 year long term effects on children? Do they have compromised immune systems?
- Of non-childbearing potential (i.e., women who have had a hysterectomy or tubal ligation or are postmenopausal, as defined by no menses in greater than or equal to 1 year).
- Of childbearing potential but has been and agrees to continue practicing highly effective contraception or abstinence (if this is the preferred and usual lifestyle of the participant) from 30 days prior to vaccination up to 6 months after the last injection (D210).
- Highly effective methods of contraception include 1 or more of the following:
- male partner who is sterile (vasectomised) prior to the female participants entry into the study and is the sole sexual partner for the female participant;
- hormonal (oral, intravaginal, transdermal, implantable or injectable);
- an intrauterine hormone-releasing system (IUS);
- an intrauterine device (IUD) with a documented failure rate of < 1%.
- A female participant is eligible if she is willing to abstain from donating oocyte from the screening visit up to 6 months after the last injection (D210);
- A male participant who is sexually active is eligible if he is willing to :
- use a condom (with/without spermicidal product) from the screening visit up to 6 months after the last injection (D210) except if the male participant is sterile (e.g. vasectomised); the unique female sexual partner is postmenopausal (defined as no menses for 12 months without an alternative medical cause), is permanently sterilized (e.g. hysterectomy or tubal ligation), or use a highly effective methods of contraception; Why? Are they concerned about something? Could this affect human reproduction?
- not donate sperm from the screening visit up to 6 months after the last injection (D210); Why? Are they concerned about something? Could this affect human reproduction?
- not plan to father a child from the screening visit up to 6 months after the last injection (D210). Why? Are they concerned about something? Could this affect human reproduction?
- Negative HIV 1/2 antibody/antigen test, Hepatitis B surface antigen (HBsAg), and Hepatitis C virus (HCV) antibody.
- Able to understand and comply with planned study procedures and willing to be available for all study-required procedures, visits and calls for the duration of the study.
- Willing to abstain from donating whole blood or blood derivatives, tissue or organ all along the study.
- Affiliated to a social security system, (except state medical aid) (Only for France).
- Volunteer registered in the French Health Ministry computerized file and authorized to participate in a clinical trial (only for France).
- Subjects actively or previously infected by SARS-CoV-2, as determined by a positive RT-PCR and positive serology test. Note: it is understandable that an INITIAL vaccine test would want non-infected/previously infected people. However, notice that studies are NOT being conducted to study Breakthrough Cases, Antibody Dependent Enhancement (ADE), and Immune Escape.
- Subject currently working with high risk of exposure to SARS-CoV-2 (e.g. health care worker, emergency response personnel, etc.) or considered at the investigator’s discretion to be at increased risk to acquire SARS-CoV-2 for any other reason. NOTE: So logically these people should definitely not take the vaccine. This definitely includes anyone except the “laptop class” who works at home and orders groceries through Amazon.
- Previous vaccination with an investigational COVID-19 vaccine. Note: where are the studies of people getting vaccinated again (e.g. lost vaccine card) or cross-vaccinated with different manufacturers? Governments are telling people they must get re-vaccinated in some cases.
- History of presence of pulmonary disorders (e.g. COPD, etc.) or asthma.
- History or present of thrombocytopenia and/or bleeding disorders.
- A positive serum pregnancy test at screening or urine pregnancy test prior to study injection, women who are planning to become pregnant during the study, or women who are breastfeeding. Not unusual for purposes of initial studies but this needs to be studied before governments start vaccinating pregnant women and children.
- Clinically relevant history of or current renal, hepatic, gastrointestinal, cardiovascular, respiratory, skin, hematological, endocrine, inflammatory, autoimmune, central nervous system or neurological diseases or clinically relevant abnormal laboratory values. Most people have some kind of issue like this. If trials weren’t done on these groups, how can governments universally vaccinate?
- Use of immunosuppressive drugs like e.g. corticosteroids (excluding topical preparations and inhalers) within 3 months prior to the first vaccination or 6 months for chemotherapies and all along the study.
- A diagnosis of schizophrenia, bipolar disease, or history of hospitalization for a psychiatric condition or previous suicide attempt. I wonder how many people this knocks out of the study? With all the psychological drug commercials on American TV stations, what portion of the population is automatically untested for this vaccine?
- A history of treatment for any other psychiatric disorder in the past 3 years that increases the risk to the subject in the opinion of the investigator.
- Received immunoglobulin or other blood product within 3 months prior to enrollment or planned receipt of immunoglobulin or a blood product through study completion.
- Vaccination within 4 weeks prior to first injection or planning to receive a licensed vaccine before D56 (e.g. Inactivated influenza vaccine). Yet governments are saying that virtually anyone can be vaccinated. Where are the tests on people with other pre-existing vaccinations? How can governments ethically push untested vaccines?
- Received measles-containing vaccine within 3 months prior to enrollment. Same as #12.
- History of severe adverse reactions to vaccine administration , including anaphylaxis and related symptoms, such as urticaria, respiratory difficulty, angioedema and abdominal pain to vaccines, or history of known or suspected allergic reaction likely to be exacerbated by any component of the COVID-19 vaccine. This is a large population, particularly those who dismissed their symptoms as “normal”.
- Participation in another investigational clinical study within four weeks before the screening visit or planned before the study completion.
- Individuals who are living and/or working with severely immunocompromised people, pregnant women, lactating women, children under 12 months old, or any other individual that, in the judgment of the investigator, might be at increased risk. So there are no studies on how vaccinated people will shed viral matter onto increased risk people. There is no proof either way of the vaccine preventing viral spread. We don’t know if the vaccinated person is somehow also spreading spike protein to others, or some other hypothetical failure mode.
- Any condition that, in the opinion of the investigator, may interfere with the aim of the study or the safety or wellbeing of the subject. It appears this study is merely paper fiction designed to push the vaccine onto everyone while claiming “it’s safe”.
- Subjects with any condition associated with, or that might be associated with, an increased risk of severe illness from COVID-19 according to US CDC definition . Again, another cherry picking example which is NOT representative of the true demographic population.
- Subjects with confirmed or suspected immunodeficiency .
- Exposure to an individual with confirmed COVID-19 or SARS-CoV-2 infection within the past 2 weeks prior to enrollment . This basically would, in the real world, exclude everyone involved in a pandemic. This is why virologists think it’s crazy to vaccinate during a pandemic.
- Subject with an acute disease and/or fever (body temperature ≥ 38°C) at the time of the 1st vaccination visit.
- History of confirmed SARS-CoV or MERS-CoV infection . Where are the re-infection studies? Breakthrough Cases, Antibody Dependent Enhancement (ADE), and Immune Escape.
- Current heavy smoker defined as smoking at least 20 cigarettes (1 pack, or equivalent) per day or former heavy smoker who was an active heavy smoker within the last year prior to the screening visit or has a total smoking history of ≥ 1 pack per day for 10 years or more. Where are the studies on smokers? How many people smoke/vape in the real world? How can governments push this product as “safe” when it hasn’t been tested on these people?
- Current or history of alcohol or drug abuse during the previous 3 years. Most Americans! Where are the studies proving it is safe for these people?
- Presence of tattoos that, in the opinion of the investigator, would preclude evaluation of the injection site.
Clinical Trials articles
| 49999 |  March 14, 2022
March 14, 2022
|
Dr. John Campbell – Ivermectin in Africa and Brazil |
| 49875 |  March 9, 2022
March 9, 2022
|
Dr. John Campbell – Withheld Pfizer Documents Released by Court Order – Significant Historic Record Breaking Safety Problem |
| 49818 |  March 5, 2022
March 5, 2022
|
FDA Releases 10,000 More Pfizer Vaccine Documents. What Will They Reveal? |
| 48936 |  February 3, 2022
February 3, 2022
|
Hidden Pfizer Covid-19 Vaccine Trial Data suggests all Pregnant Vaccinated Women Miscarried |
| 48872 |  February 1, 2022
February 1, 2022
|
The Pfizer Inoculations for COVID-19 – More Harm Than Good |
| 48242 |  December 30, 2021
December 30, 2021
|
More Harm Than Good – The Pfizer Inoculations For COVID-19 |
| 47031 |  December 15, 2021
December 15, 2021
|
Joe Rogan Experience #1747 – Dr. Peter McCullough (with video) |
| 44957 | November 30, 2021 | Covid-19 Vaccine Clinical Trials: Failure to Properly Assess Safety and Efficacy |
| 44877 | November 29, 2021 | FDA Asks Court to Delay Full Release of Documents on Pfizer COVID Biologic for 55 Years |
| 44611 |  November 27, 2021
November 27, 2021
|
The 90% Mythical Pfizer/Moderna Unicorns – David Martin |