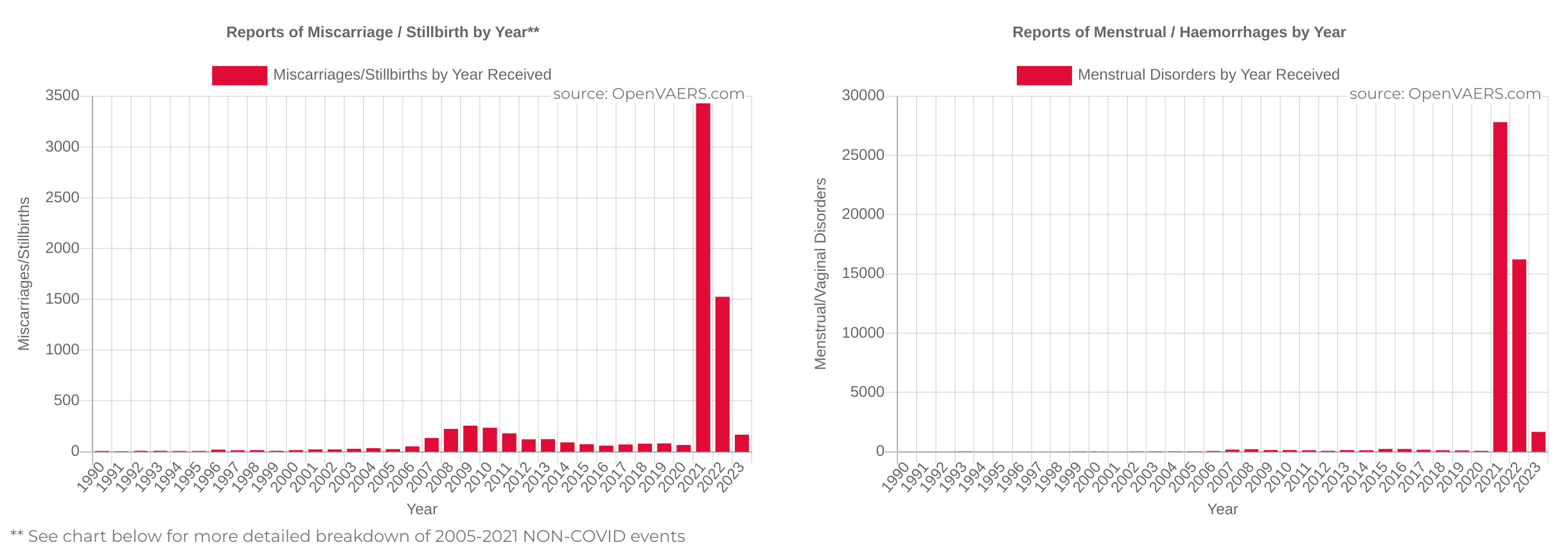
Miscarriages and Stillbirths Due to “Vaccines” Up 5,271% in 2021 Compared to 2020
By J.D. Rucker • Jun. 29, 2023 “Pregnant women should get vaxxed,” they said. The Covid-19 “vaccines” have proven to be the most dangerous and falsely promoted government-backed drugs in the history of mankind. The death tolls continue to rise, and despite unambiguous evidence that they’re neither safe nor effective, they’re still being heavily promoted as such. Corporate … Read more