https://www.rwmalonemd.com/about-us
Robert W. Malone, M.D., M.S.
Dr. Malone is the discoverer of in-vitro and in-vivo RNA transfection and the inventor of mRNA vaccines, while he was at the Salk Institute in 1988.
Update: The left has been on a censorship crusade, trying to block people from knowing he is the inventor of the mRNA technology. Leftists are now even denying that he invented the mRNA vaccine, citing instead a CEO of a big-pharma company.
His research was continued at Vical in 1989, where the first in-vivo mammalian experiments were designed by him. The mRNA, constructs, reagents were developed at the Salk institute and Vical by Dr. Malone. The initial patent disclosures were written by Dr. Malone in 1988-1989. Dr. Malone was also an inventor of DNA vaccines in 1988 and 1989. This work results in over 10 patents and numerous publications, yielding about 7000 citations for this work. Dr. Malone was also an inventor of DNA vaccines in 1988 and 1989.
Dr. Malone has extensive research and development experience in the areas of pre-clinical discovery research, clinical trials, vaccines, gene therapy, bio-defense, and immunology. He has over twenty years of management and leadership experience in academia, pharmaceutical and biotechnology industries, as well as in governmental and non-governmental organizations.
Dr. Malone specializes in clinical research, medical affairs, regulatory affairs, project management, proposal management (large grants and contracts), vaccines and biodefense. This includes writing, developing, reviewing and managing vaccine, bio-threat and biologics clinical trials and clinical development strategies. He has been involved in developing, designing, and providing oversight of approximately forty phase 1 clinical trials and twenty phase 2 clinical trials, as well as five phase 3 clinical trials. He has served as medical director/medical monitor on approximately forty phase 1 clinical trials, and on twenty phase 2 clinical trials, including those run at vaccine-focused Clinical Research Organizations. His proposal development work has yielded clients billions of dollars.
Scientifically trained at UC Davis, UC San Diego, and at the Salk Institute Molecular Biology and Virology laboratories, Dr. Malone is an internationally recognized scientist (virology, immunology, molecular biology) and is known as one of the original inventors of mRNA vaccination and DNA Vaccination. His discoveries in mRNA non viral delivery systems are considered the key to the current COVID-19 vaccine strategies. Dr. Malone holds numerous fundamental domestic and foreign patents in the fields of gene delivery, delivery formulations, and vaccines.
He received his medical training at Northwestern University (MD) and Harvard University (Clinical Research Post Graduate) medical school, and in Pathology at UC Davis.
Dr. Malone has close to 100 peer-reviewed publications and published abstracts and has over 11,477 citations of his peer reviewed publications, as verified by Google Scholar. His google scholar ranking is “outstanding” for impact factors. He has been an invited speaker at over 50 conferences, has chaired numerous conferences and he has sat on or served as chairperson on numerous NIAID and DoD study sections.
Articles and Videos
-
Rand Paul Says Resist COVID Medical Tyranny
https://www.bitchute.com/video/lzQPEwbfEvol/Continue reading »
-
Immunologist gives testimony to Indiana School District – CDC ineffective, masks don’t work, use Ivermectin (repurposed drugs)
https://rumble.com/embed/vicn8p/?pub=5zpyl [sc name="fullarticle" ]https://rumble.com/vkytdz-medical-expert-annihilates-school-board-over-unscientific-mask-mandates.html[/sc]Continue reading »
-
Number of Deaths Reported After COVID Vaccines Jumps by More Than 2,000 in 1 Week, According to VAERS
https://childrenshealthdefense.org/defender/cdc-vaers-deaths-reported-covid-vaccines/?utm_source=salsa&eType=EmailBlastContent&eId=7fa4238d-0294-400f-b083-ed7320af3dc7Continue reading »
-
Dr. Fauci Predicted a Pandemic Under Trump in 2017
https://www.youtube.com/watch?v=puqaaeLnEww “And if there’s one message that I want to leave with you today based on my experience … is…Continue reading »
-
Navy Doctor Persecuted by Biden Era DOJ For Injecting With Saline
[sc name="sourcelink" ]https://revolver.news/2025/07/mtg-throws-hail-mary-for-navy-doc-facing-life-in-prison-for-saying-no-to-bidens-vax-madness/[/sc] During some of the darkest days in American history, when COVID fear ruled the airwaves and mandates…Continue reading »
-
Charlene Carter’s (Southwest Airlines flight attendant) Religious Freedom Case
Read the court case: [pdf-embedder url="https://mymedicalfreedom.org/wp-content/uploads/2025/07/Charline-Carter-court-case.pdf" title="Charline Carter court case"] Download PDF A Grok summary which may contain errors: ###…Continue reading »
-
Stephen Colbert’s Vomit-Inducing “The Vax-Scene” (Remastered to 4K/60fps)
RumbleContinue reading »
-
Dr. Patrick Soon-Shiong: You’re Being Lied to About Cancer, How It’s Caused, and How to Stop It
on RumbleContinue reading »
-
Airline Employees 4 Health Freedom
[sc name="sourcelink" ]https://aerocrewnews.com/2025/03/01/airline-employees-4-health-freedom/[/sc] By Capt. Laura Cox - March 1, 2025 Written by: Capt. Laura Cox and Capt. Sherry Walker…Continue reading »
-
Norway Sounds Alarm as Scientists Link Covid ‘Vaccines’ to Global Death Surge
[sc name="sourcelink" ]https://slaynews.com/news/norway-sounds-alarm-scientists-link-covid-vaccines-global-death-surge/[/sc] Frank Bergman February 6, 2025 - 12:58 pm A group of leading scientists in Norway is sounding…Continue reading »
-
BOMBSHELL: Co-Founder and CEO of BioNTech Refuses to Take the mRNA Covid Vaccine Because He says, “We Need to Ensure Functionality of Our Whole Company”
[sc name="sourcelink" ]https://www.infowars.com/posts/bombshell-co-founder-and-ceo-of-biontech-refuses-to-take-the-mrna-covid-vaccine-because-he-says-we-need-to-ensure-functionality-of-our-whole-company[/sc] by S.D. Wells | Natural NewsJanuary 8th, 2025 7:01 AM Imagine if an automobile manufacturer recommended their…Continue reading »
-
IVERMECTIN and CANCER, it has at least 15 anti-cancer mechanisms of action. Can Ivermectin Treat COVID-19 mRNA Vaccine Induced Turbo Cancers? – 9 Ivermectin papers reviewed
[sc name="sourcelink" ]https://makismd.substack.com/p/ivermectin-and-cancer-it-has-at-least[/sc] Dr. William Makis MD Oct 02, 2023 ∙ Paid Papers reviewed: 2023 Sep.23 - Man-Yuan Li et al -…Continue reading »
-
Fenbendazole Cancer Success Stories: 83 Case Reports Compilation (January 2025 Edition)
[sc name="sourcelink" ]https://www.onedaymd.com/2024/02/fenbendazole-cancer-success-stories.html[/sc] [pdf-embedder url="https://mymedicalfreedom.org/wp-content/uploads/2025/01/Fenbendazole-Cancer-Success-Stories_-83-Case-Reports-Compilation-January-2025-Edition.pdf" title="Fenbendazole Cancer Success Stories_ 83 Case Reports Compilation (January 2025 Edition)"]Continue reading »
-
U.S. House of Representatives Report on Coronavirus Pandemic
[sc name="sourcelink" ]https://oversight.house.gov/wp-content/uploads/2024/12/2024.12.04-SSCP-FINAL-REPORT-ANS.pdf[/sc] [pdf-embedder url="https://mymedicalfreedom.org/wp-content/uploads/2025/01/2024.12.04-SSCP-FINAL-REPORT-ANS.pdf"]Continue reading »
The True Story of How mRNA Vaccination was Invented

IT ALL STARTED WHEN…
Dr. Malone is the inventor of mRNA vaccines (and DNA vaccines). He also discovered lipid mediated and naked RNA transfection technologies.
It all started when he was at the Salk Institute in 1987 and 1988. There, he pioneered in-vitro RNA transfection and also in-vivo RNA transfection (in frog embryos, as well as mice).
This resulted in his seminal paper: Cationic liposome-mediated RNA transfection RW Malone, PL Felgner, IM Verma. Proceedings of the National Academy of Sciences (PNAS) 86 (16), 6077-6081
His filed patent and disclosures from the Salk included in-vivo RNA transfection and also methods for mRNA stabilization – now being claimed as invented by others. These are available for review.
His research was continued at Vical in 1989, where the first in-vivo mammalian rat experiments were designed by him. The mRNA, constructs, reagents were developed at the Salk institute and at Vical by Dr. Malone, this included dosing amounts for the in-vivo experiments. RNA and DNA were sent to Dr. Jon Wolff via Fedex. Dr.Wolff at the University of Wisconsin injected mice and rats. The initial patent disclosures for RNA and DNA vaccination were written by Dr. Malone in 1988-1989. Dr. Malone was also an inventor of DNA vaccines in 1988 and 1989.
This body of work resulted in over 10 patents and numerous publications, yielding about 7000 citations for this work. The first paper is:
Direct gene transfer into mouse muscle in vivo. Wolff JA, Malone RW, et al. Science. 1990;247(4949 Pt 1):1465-8. Cited in 4,750 articles, is the result of that work.
In 1989, research was performed that gave rise to the 10+ groundbreaking patents on mRNA vaccination, all with a priority date of March 3, 1989. This is the same priority date as the Salk Patent application, showing that the two institutions were working together (without Robert’s knowledge). These patents are the first published research on mRNA vaccination. The titles and links to the patents are listed in the documents below.
Vical was to license the Salk Technology. Instead, they hired Robert’s thesis advisor from the Salk and soon after, the Salk dropped the patent and Vical never pursued a license from the Salk. Due to an employee contract with Vical, this stopped Robert from working in the field commercially for a decade. Vical claimed all the Salk research happened at Vical and sent a cease and desist letter.
Dr. Malone carried on his research into mRNA vaccination during the 1990s, culminating in a mucosal patent that was issued in 2000. He also helped revolutionized the field of cationic liposomes for the use in RNA vaccinations. This work was so far ahead of its time, that only now is the world turning to mucosal mRNA vaccination as a method of immunization. For a listing of some of his work, see the publications at the end of this page.
Scientifically trained at UC Davis, UC San Diego, and at the Salk Institute Molecular Biology and Virology laboratories, Dr. Malone received his medical training at Northwestern University (MD) and Harvard University Medical School (Clinical Research Post Graduate) , and in Pathology at UC Davis, He has almost 100 peer-reviewed publications, and has been an invited speaker at about 50 conferences.
For the full backstory on the discovery of mRNA vaccination, as written by Dr. Malone’s partner and wife, please download the PDF below:

Image from Heidi News
For an article on Dr. Malone’s discoveries,
click on the title below (published in Heidi.news)
Intellectual Rape at the Salk Institute
mRNA Vaccine and RNA Transfection/Delivery Papers and Patents directly derived from Robert W Malone’s research
The Vical patent that was filed with the USPTO on 3/21/1989. Note that the cover letter hides this – and says it was filed on 3/29/89.
THE VERY FIRST mRNA VACCINE EXPERIMENTAL DATA 1990 (from Vical to patent office)
DNA and RNA Transfection and Vaccination (Abstract). First Place, Northwestern AOA Research Symposium Competition for Medical Students: 1989.
Cationic liposome-mediated RNA transfection. Malone RW, Felgner PL, Verma IM. Proc Natl Acad Sci (PNAS) U S A. 1989;86(16):6077-81. Cited in 749 articles.
Direct gene transfer into mouse muscle in vivo. Wolff JA, Malone RW, et al. Science. 1990;247(4949 Pt 1):1465-8. Cited in 4,750 articles. Note that I was a student at Northwestern, and was never affiliated with University of Wisc.
High levels of messenger RNA expression following cationic liposome mediated transfection tissue culture cells. Malone R, Kumar R, Felgner P. NIH Conference: “Self-Cleaving RNA as an Anti-HIV Agent (abstract). Washington, DC June 1989.
Cationic liposome-mediated RNA transfection. Dwarki VJ, Malone RW, Verma IM. Methods Enzymol. 1993;217:644-54. Cited in: 102 articles.
Delivery of exogenous DNA (includes mRNA) sequences in a mammal P Felgner, JA Wolff, GH Rhodes, R Malone, D Carson. Biotechnology Advances 1993: 15 (3-4), 763-763
Lipid-mediated polynucleotide administration to deliver a biologically active peptide and to induce a cellular immune response (includes mRNA). P Felgner, JA Wolff, GH Rhodes, R Malone, D Carson. Assigned to Vical, Inc and licensed to Merck. No. 7,250,404, date of issue: 7/31/07 Cited in 105 articles. Priority Date: 3/21/1989.
Lipid-mediated polynucleotide administration to reduce likelihood of subject’s becoming infected (includes mRNA). P Felgner, JA Wolff, GH Rhodes, Robert W Malone, D Carson. Assigned to Vical, Inc and licensed to Merck. US Pat. Ser. No. 6,867,195 B1. Date of issue: 3/15/05. Priority Date: 3/21/1989.
mRNA vaccination experiment found in mRNA vaccine patents, with a priority date of 1989. This example comes from patent #6,867,195.
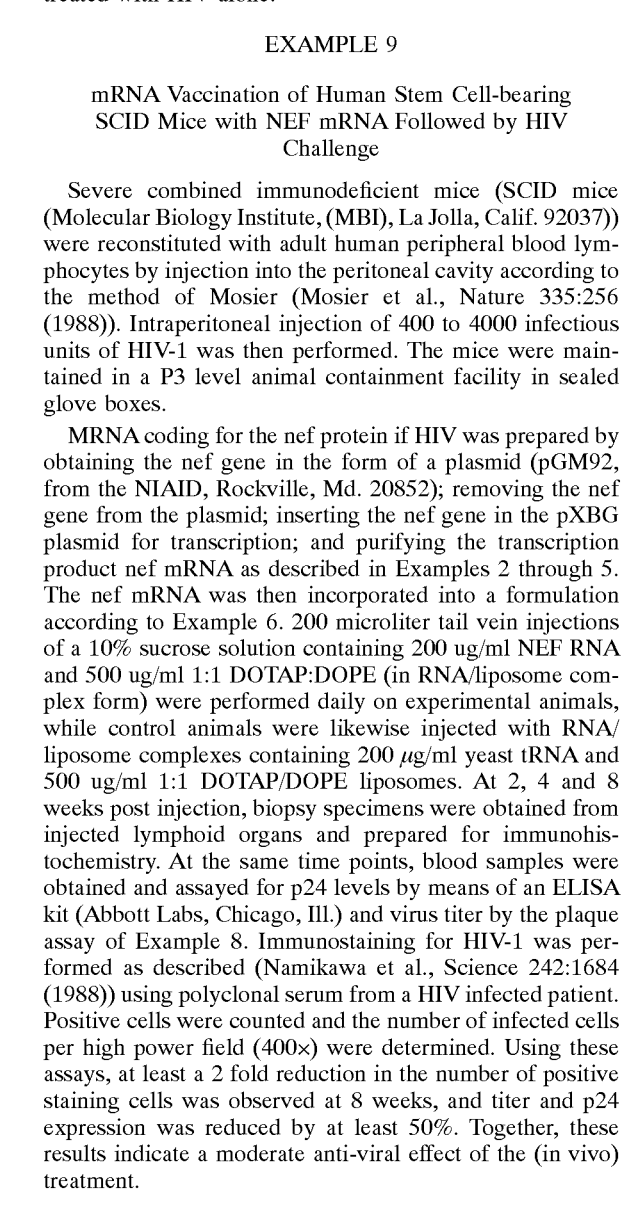
Generation of an immune response to a pathogen (includes mRNA). P Felgner, JA Wolff, GH Rhodes, Robert W Malone, D Carson. Assigned to Vical, Inc and licensed to Merck. US Pat. Ser. No. 6,710,035. Date of issue: 3/23/04. Citations: 39 articles. Priority Date: 3/21/1989.
Cationic Transport Reagents. US Pat. Ser. No. 5,892,071 Robert W Malone, et. al. issued 4/06/99.
Cationic Transport Reagents. Robert W Malone, et. al. US Pat. Ser. No. 5,744,625 issued 4/28/98.