What is VAERS
Established in 1990, the Vaccine Adverse Event Reporting System (VAERS) is a national early warning system to detect possible safety problems in U.S.-licensed vaccines. VAERS is co-managed by the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA). VAERS accepts and analyzes reports of adverse events (possible side effects) after a person has received a vaccination. Anyone can report an adverse event to VAERS. Healthcare professionals are required to report certain adverse events and vaccine manufacturers are required to report all adverse events that come to their attention.
VAERS is a passive reporting system, meaning it relies on individuals to send in reports of their experiences to CDC and FDA. VAERS is not designed to determine if a vaccine caused a health problem, but is especially useful for detecting unusual or unexpected patterns of adverse event reporting that might indicate a possible safety problem with a vaccine. This way, VAERS can provide CDC and FDA with valuable information that additional work and evaluation is necessary to further assess a possible safety concern.
VAERS Reporting Rates (“underreporting”)
A 2011 report by Harvard Pilgrim Health Care, Inc. for the U.S. Department of Health and Human Services (HHS) stated that fewer than one percent of all vaccine adverse events are reported to the government:4
r18hs017045-lazarus-final-report-2011Although 25% of ambulatory patients experience an adverse drug event, less than 0.3% of all adverse drug events and 1-13% of serious events are reported to the Food and Drug Administration (FDA). Likewise, fewer than 1% of vaccine adverse events are reported. Low reporting rates preclude or slow the identification of “problem” drugs and vaccines that endanger public health. New surveillance methods for drug and vaccine adverse effects are needed.
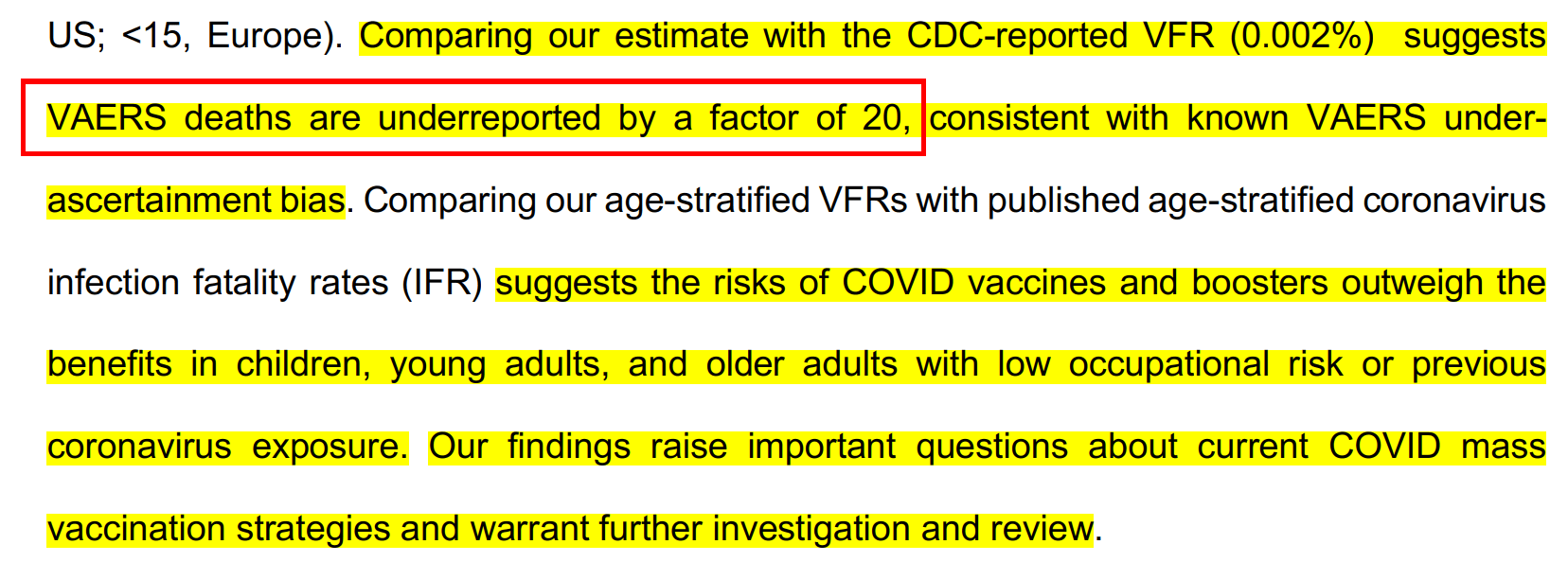
Study Shows VAERS deaths are underreported by a factor of 20
See: New Columbia University “Vaccine” Study Drops Bombshell: VAERS Deaths Undercounted by Factor of 20
Required and Optional VAERS Reporting
- Healthcare providers are required by law to report to VAERS:
- Any adverse event listed in the VAERS Table of Reportable Events Following Vaccination that occurs within the specified time period after vaccinations.
- An adverse event listed by the vaccine manufacturer as a contraindication to further doses of the vaccine
- Healthcare providers are strongly encouraged to report to VAERS:
- Any adverse event that occurs after the administration of a vaccine licensed in the United States, whether it is or is not clear that a vaccine caused the adverse event
- Vaccine administration errors
- Manufacturers are also required by regulation (21 CFR 600.80) to report to the VAERS program all adverse events made known to them for any vaccine.
Counterargument to VAERS
False Reporting to VAERS
- Knowingly filing a false VAERS report is a violation of Federal law (18 U.S. Code § 1001) punishable by fine and imprisonment.
V-SAFE vs. VAERS
- There is a parallel system in place that most people are told to use.
- It’s called V-SAFE.
- The CDC appears to be completely unresponsive to releasing the data collected via this system.
- CDC claims V-SAFE data is fed into VAERS but this is apparently not raw data but instead being processed by the CDC.
