Highlights
- •
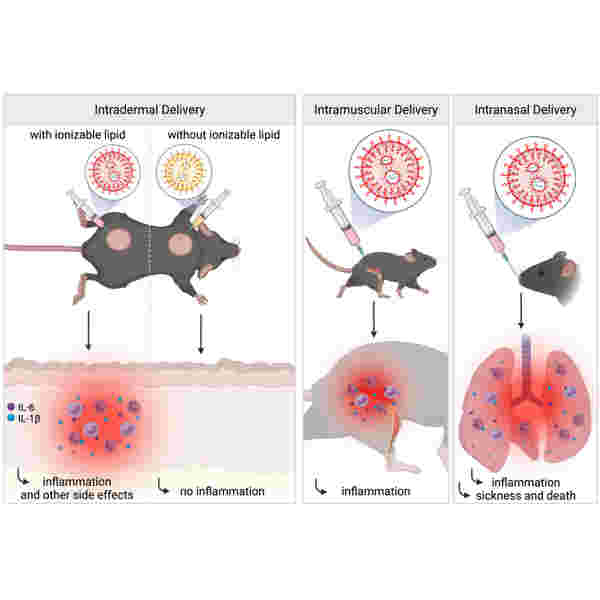
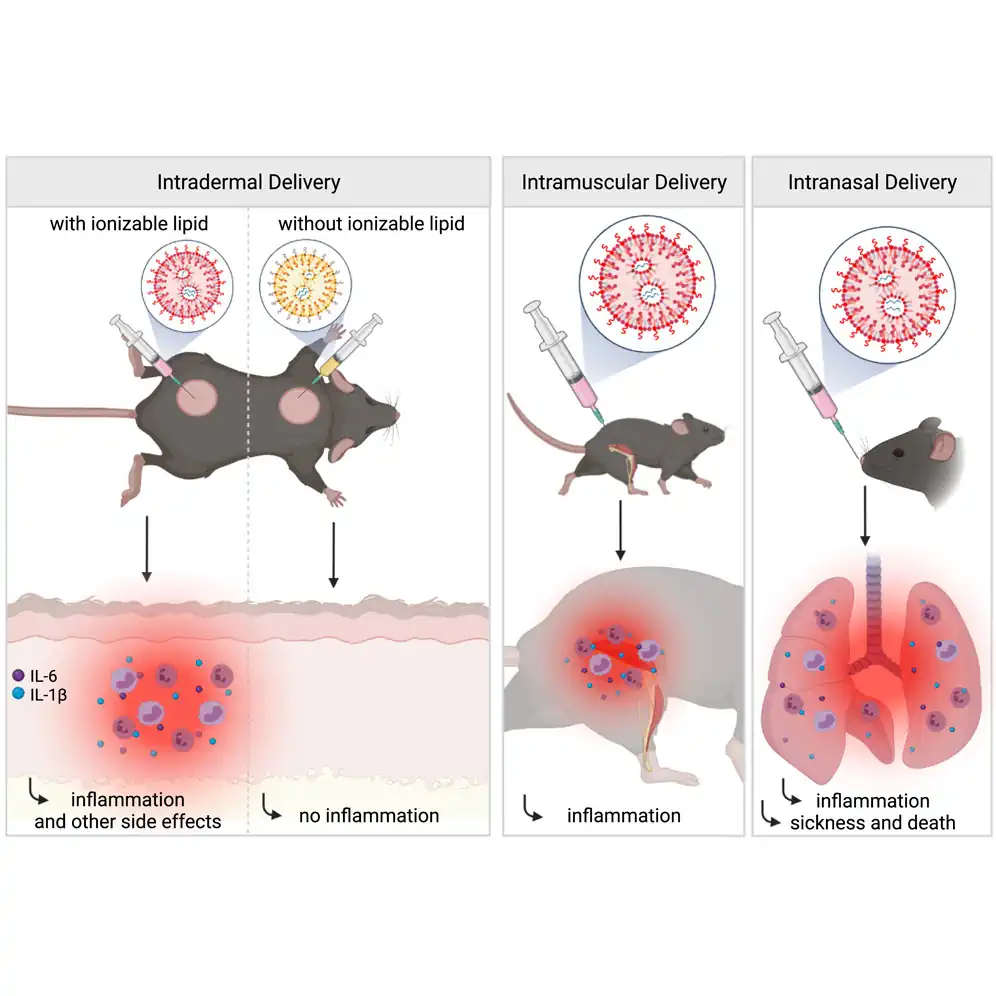
Lipid nanoparticles (LNPs) used for preclinical studies are highly inflammatory
- •
The LNPs activate multiple inflammatory pathways and induce IL-1β and IL-6
- •
The LNPs’ inflammatory properties stem from their ionizable lipid component
- •
The LNPs could be responsible for adjuvanticity and some of the side effects
Summary
Vaccines based on mRNA-containing lipid nanoparticles (LNPs) are a promising new platform used by two leading vaccines against COVID-19. Clinical trials and ongoing vaccinations present with varying degrees of protection levels and side effects. However, the drivers of the reported side effects remain poorly defined. Here we present evidence that Acuitas’ LNPs used in preclinical nucleoside-modified mRNA vaccine studies are highly inflammatory in mice. Intradermal and intramuscular injection of these LNPs led to rapid and robust inflammatory responses, characterized by massive neutrophil infiltration, activation of diverse inflammatory pathways, and production of various inflammatory cytokines and chemokines. The same dose of LNP delivered intranasally led to similar inflammatory responses in the lung and resulted in a high mortality rate, with mechanism unresolved. Thus, the mRNA-LNP platforms’ potency in supporting the induction of adaptive immune responses and the observed side effects may stem from the LNPs’ highly inflammatory nature.