All COVID-19 Vaccine Studies Used nonQ-RT-PCR to determine case status. All of the estimates of outcome are unreliable. This is the most important study we will ever likely publish in our journal.
NB: The toy math example to show how calculations of False Discovery Rate lead to bias in favor of false positives, an error has been corrected. Corrections and changes are in bold. The original article references to ‘false positive rate’ will also be updated to ‘false discovery rate’. We thank our readers for catching those errors!
We have just published a new study that shows that nonQ-RT-PCR (non-quantitative RT-PCR testing as used to diagnose COVID-19 from 2020 to the present day suffers a flaw that ultimately draws into question all of what has been reported on COVID-19 by official channels, including the results of COVID-19.
Specifically, assuming a 5% prevalence rate, the high false discovery rate (42%) of the use of nonQ-RT-PCR means
1. For every 50 true positives out of 1,000, a total of 86 people with or without SARS-CoV-2 infection or residual fragments will be reported. Of these, 36 of these will be false positives.
2. For every 50 true positives, 86 people without SARS-CoV-2 infection or residual fragments will be have to be isolated/quarantined. Of these, 36 will not be infected.
3. For every 50 true positives that are tested and found positive in-hospital, 86 people with or without SARS-CoV-2 infection or residual fragments will be told that they “have COVID-19”. If the 36 false positive patients are hospitalized with other COVID-19 patients, they will likely then contract a SARS-CoV-2 infection.
4. The number of “cases” via positive PCR has been overstated by a factor of 72% (the original post read “80:1” assuming a prevalence of 5%).
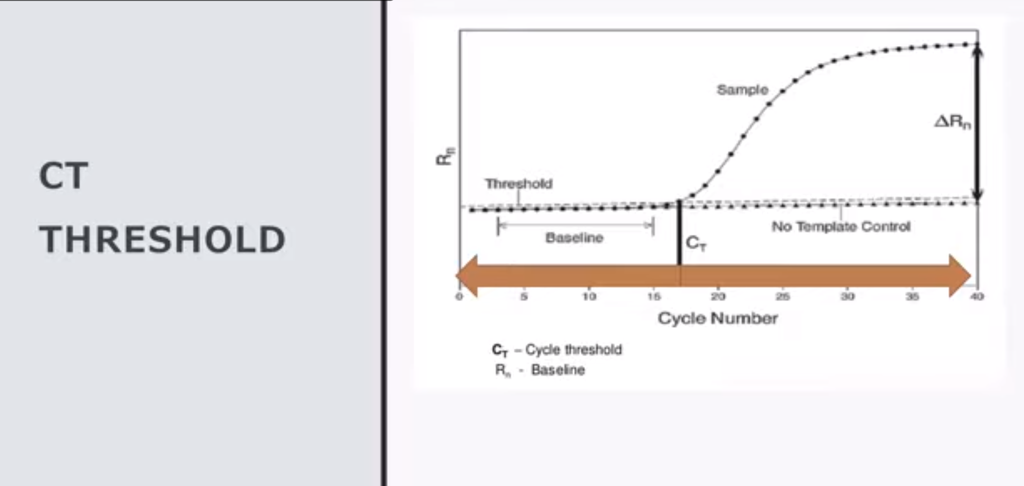
5. This is true for generic case reporting up until May 2021 when CDC decided to reduce the PCR cycle threshold value (Ct) for the vaccinated to less than 27, leaving the unvaccinated rate biased by high false discovery rate of arbitrarily high Ct, biasing all reported rates in these two groups favoring cases in the unvaccinated from that point on.
6. This +72% bias is true in any clinical trial or any study that used arbitrarily high Ct values, INCLUDING THE VACCINE STUDIES.
As a direct result of this fatal flaw, combined with CDC’s gaff “PCR+ = COVID-19″?
There are no credible COVID-19 vaccine trial data.
In 2003, CDC took the credit for curtailing the SARS-CoV-1 transmission. Among the method of control they claimed were essential to this included SARS-CoV-1 strain-specific PCR primers used to produce amplicons that were sequenced. The presence of the sequence was used to infer, correctly, whether the PCR reaction had produced a population of SARS-CoV-1 DNA molecules that were sequenced using FDA-designated gold standard – Sanger Sequencing, or an arbitrary population of DNA molecules that represented off-target amplicons.
In 2020, for reasons no one has ever explained, the CDC changed the nucleic acid detection protocol to one that had never been tried before for control of respiratory viruses. Instead of using sequence-based detection, they merely used the results of a non-quantitative reverse transcriptase (RT)-PCR as evidence of the presence of the virus, and then, equally inexplicably, decided to determine that a positive nonQ-RT-PCR test result indicated disease (COVID-19).
Anyone trained in nucleic assays would know this would lead to excessive false positives. Somehow, per official narrative, zero false positive test results were expected by CDC – even with their own test, which had an arbitrarily high Ct cutoff of 40.

Two scientists – Dr. James Lyons-Weiler and Dr. Sin Han Lee – were early voices in the spring of 2020 who independently were trying to alert the FDA and CDC to the gravity of the problem of potentially high false positives in SARS-CoV-2 testing. After discussing the problem, and being approached by others, including Dr. H. Ealy, and concerned citizens especially Alix Meyer of California Children’s Health Defense, Dr. Lyons-Weiler and the group created NAATEC – the Nucleic Acid Assay Technology Evaluation Consortium.
Dr. Lee had previously self-funded a study that demonstrated false positives and false negatives were occurring. Other studies were published showing false positives, or results that could only be explained by false positives.
On the steps of the Pennsylvania Capitol building, Dr. Lyons-Weiler warned that false positive tests leading to quarantine would act like a “bomb, after bomb, after bomb” going off on American society and could lead to the destruction of the US economy.
Dr. Lee’s technical (not clinical) demonstration provided sufficient evidence for the testing to be abandoned as a criterion for employment. But nonQ-RT-PCR based testing continued, and Sanger sequencing-based testing was not adopted.
Dr. Lee’s laboratory was therefore funded to, independent of any direction or scientific influence by NAATEC, to conduct a second study to extend the results to characterize what RT-PCR based diagnoses were doing in a world with SARS-CoV-2 variants.
After extensive and thorough blinded peer-review, the journal Science, Public Health Policy & the Law has now published Dr. Lee’s work.
This work is some of the finest of Dr. Lee’s career. In this study, Dr. Lee not only reports that he has verified false positives due to the misapplication of RT-PCR testing:
“PCR was invented to replicate, or to amplify, a target segment of DNA for DNA sequencing without going through a laborious bacterial cloning. PCR needs a pair of primers, single-stranded DNAs of about 20 bases long, to define the segment of target DNA to be replicated. But PCR primer/template hybridization is not fully sequence-specific because PCR primers may attach to nontarget DNAs and amplify unwanted DNAs if these DNAs are present and partially match the primers in nucleotide sequence. As a result, relying on PCR, especially the qPCR technology using Ct numbers as the surrogate for actual PCR product analysis, for disease diagnosis is bound to generate false positives. The experimental results of this work emphasize that while RT-qPCR is generating a significant number of false-positive test results at the current stage of the COVID-19 pandemic…”
He also provides this harrowing conclusion:
“The COVID-19 pandemic could have been avoided or curtailed by using the SARSCoV-1 specific RT-PCR primers in early 2020.”
Why This Matters 1: The Clinical Mess
These results demonstrate that as a direct result of the decision to change diagnostic methodology for SARS-CoV-2, hospital beds filled with patients who do not have COVID-19, but who think they have COVID-19. Other respiratory ailments such as RSV, influenza, bacterial pneumonia, fungal infections, other coronaviruses and the common cold are likely mixed in with COVID-19 patients.
The clinical mess that results is, of course that patients without COVID-19 but with other serious respiratory illnesses are receiving the wrong treatment and are dying from pneumonia unrelated to COVID-19 (PUTC).
The clinical mess also can lead to SARS-CoV-2 infections acquired in the hospital.
The problem compounds: when the prevalence the disease is low, most positive PCR tests will be negative. With a 42% false discovery rate applied to 1000 people, 5% of whom are infected, only 50 positive tests can be true positives, but an expected 36 will be false positives. Under CDC’s “infection = disease” paradigm, 36 people without SARS-CoV-2 infections have to be quarantined for every 50 true infections.
Combined then with the HHS’s “go home and do nothing until you’re sick enough for emergency care”, the 50 infected people incubate virus, create variants, leading to severely ill COVID-19 patients.
But the 36 who went home to wait for 10 days to get seriously ill also might have benefited from more refined diagnosis of their actual conditions.
They will also show up in the hospitals – and the hospitals will benefit from the perverse incentives in place per COVID-19 diagnosis.
This, of course, is deplorable.
Why This Matters 2: The Result of COVID-19 Vaccine Clinical Trials Used nonQ-RT-PCR
The published efficacy of SARS-CoV-2 vaccines, the published breakthrough rate, the published re-infection rate estimates – all of it is potentially rubbish given the 42% false discovery rate. No credible data exist on the efficacy or real-world effectiveness of SAR-CoV-2 targeting vaccines.
The impact of this error by CDC and FDA on our society has been profound, individually and collectively.
Dr. Lee’s paper cites both CDC and FDA’s decision in 2003 to use Sanger sequencing as the gold-standard check of the PCR amplicons. The reasons why this was abandoned must be examined by a Senate hearing. FDA has designated sequencing a “complex” procedure, and yet Dr. Lee reminds us that any hospital can conduct Sanger sequencing.
“To reaffirm this gold-standard approach to diagnose RNA viruses, the Food and Drug Administration (FDA) also issued a guideline on January 2, 2009 that detection of enterovirus RNA requires generating RT-PCR amplicons from two different genomic regions of the virus and to perform bi-directional sequencing on one of the amplicons; and the sequence of the amplicon should match the reference or consensus sequence of the virus.”
Dr. Lee’s test is so accurate it can be used to overrule RT-PCR and antigen tests. Unlike many others, however, Dr. Lee does not wish to become a testing center or a billionaire with his test.
In fact, in his study, he advises that hospitals develop their own Sanger sequencing testing capacity and not wait for FDA approval. Dr. Lee has discovered a set of primers every hospital can use, and has published them in the report.
“A set of RT-PCR primers targeting a highly conserved genomic segment of SARS coronaviruses, such as the CDC-recommended SARS-CoV-1 specific RT-PCR primers [10] or the N gene RT-PCR primers presented in this paper, should be available to all major community hospital laboratories in the world in preparation for a timely accurate diagnosis in the next SARS coronavirus outbreak. The hospital laboratories dealing with patients should not wait for the commercial companies to develop an approved test kit to diagnose another emerging SARS coronavirus for early patient treatment and isolation.”
This new study goes beyond anything any CDC or hospital lab has done to evaluate RT-PCR testing as allowed by FDA under the EUAs given for such tests.
Dr. Lee used sequence-level data such as this figure:
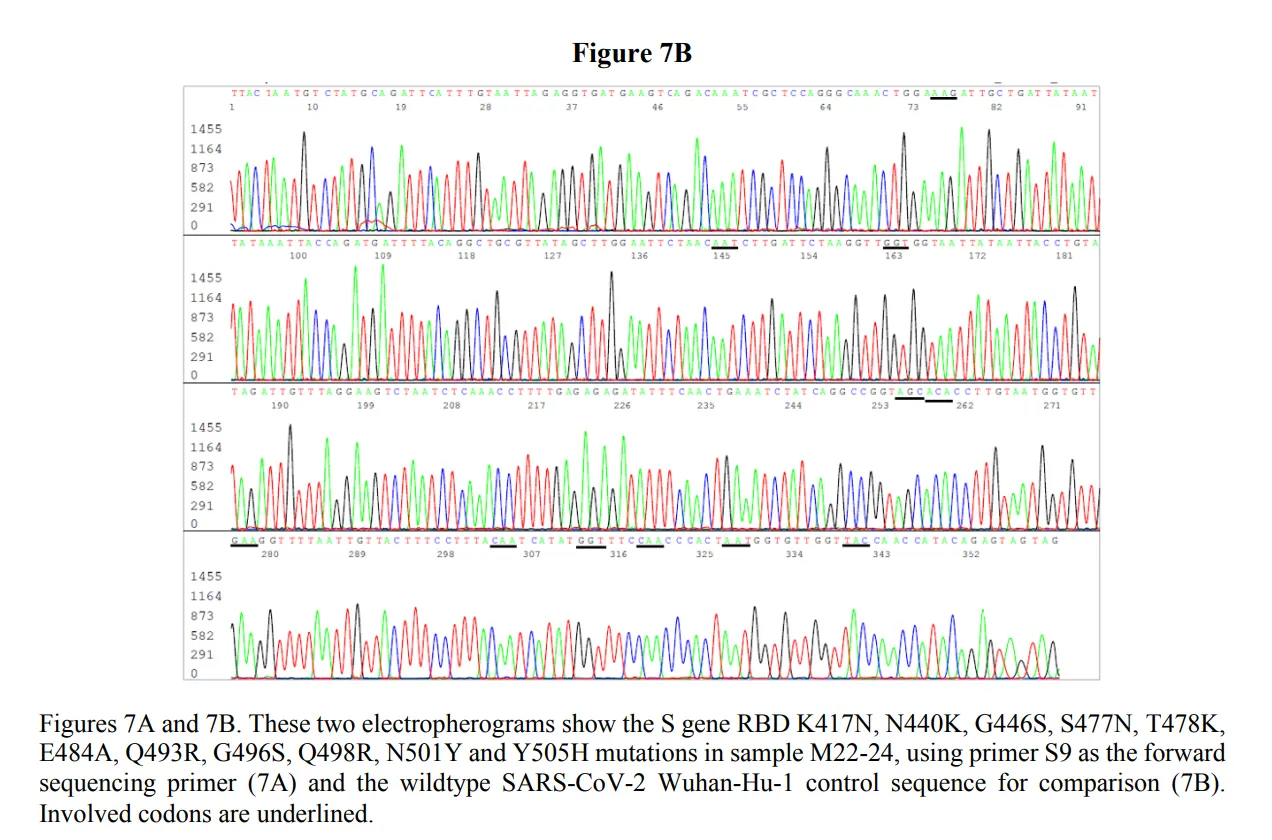
to study specific mutations in sequences generated using general primers that would detect both SARS-CoV-1 and SARS-CoV-2. The resulting sequences of the amplicons should have been used to study and understand the actual presence and absence of SARS-CoV-2. This approach, instead of ambiguous SARS-CoV-2 targeting primers that lead to high false positives, would have prevented wasted resources on people who did not have SARS-CoV-2.
Why This Matters 3: Diffuse, Ongoing Personal Lockdowns
Your Congressmen, Senators, School Boards, Employers, health departments and your friends and family deserve to know and understand that their RT-PCR test results – and those of their loved ones – may, in fact, be incorrect, and that the CDC protocol has an unacceptably high false discovery rate. Isolation due to false positives is disruptive caused mass chaos in all sectors of society, and false positives gave – and are still giving – individual patients a misunderstanding of their immune and infectious status.
Dr. Lee’s earlier published study showing false negatives in the nonQ-RT-PCR designated technical samples also means that people have been testing negative for SARS-CoV-2 infection when, in fact, they had a SARS-CoV-2 virus infection.
Dr. Lee, Dr. Lyons-Weiler, and Dr. Ealy tried in earnest to tell the FDA and CDC all of this would happen. They were able to see the most fundamental details of mass-testing, and, as a result, the cost to society has been immeasurable.
The response by CDC was to lower the Ct threshold for case determination in the vaccinated in May of 2021, but to continue biasing the unvaccinated case count upward using arbitrarily high Ct threshold values in the unvaccinated.
It’s time to demand accountability and bring public health to heel, answerable to elected, not appointed officials.
Study Citation
Lee, SH. 2022. Evidence-Based Evaluation of PCR Diagnostics for SARS-CoV-2 and the Omicron Variants by Sanger Sequencing. Science, Public Health Policy & the Law 4: Open Access: https://www.publichealthpolicyjournal.com/about-7
22-11-LeeFDR