Researchers suspect “immune imprinting” is responsible.
Less than two months after the FDA used mouse data to give emergency use authorization to bivalent COVID-19 boosters, the real-world performance of the shots that target the ancestral strain and Omicron BA.4/5 subvariants is undermining the feds’ one-size-fits-all messaging.
New preprint studies by researchers at Columbia University and Beth Israel Deaconness Medical Center, not yet peer-reviewed, found that the bivalents showed no meaningful improvement over the original monovalent boosters that targeted the Wuhan strain alone.
CDC Director Rochelle Walensky got infected by COVID this past weekend, just a month after taking her bivalent booster. The new boosters are “formulated to better protect against” newer variants, she said when recommending them in September.
“Ironically, this is probably the peak vaccine efficacy (peak Ab),” University of California San Francisco epidemiologist Vinay Prasad wrote in his newsletter Wednesday, referring to Walensky’s ill-timed breakthrough.
“Their entire vaccine policy seems to be interested in giving Pfizer and Moderna a perpetual market share for a yearly vaccine,” he said. “But seems to have no interest in generating credible randomized control trial evidence to inform the public.”
“[A]nyone billing these bivalent vaccines as ‘better’ or ‘improved’ is simply not telling the truth,” Hawaii family physician Buzz Hollander wrote in his newsletter, which was picked up by RealClearScience.
“So let’s not pretend; because if the Wuhan/BA.4-5 bivalent data ends up as mediocre as the early returns on the Wuhan/BA.1 booster, people are going to wonder why they were sold a ‘better’ product after so little study,” he said.
Human data on Pfizer's bivalent booster!
Kind of.https://t.co/t7G47ptOq3
At 1 wk, Wuhan booster in 40 ppl>55 "elicited more limited increases" in nAb vs bivalent. Interesting phrasing🧐
Assume results not dramatic.
Saw this at 1 mo w/ BA1/Wuhan bivalent, & its VE was no better. pic.twitter.com/VQ0b5CFRek— Buzz Hollander MD (@buzzhollandermd) October 13, 2022
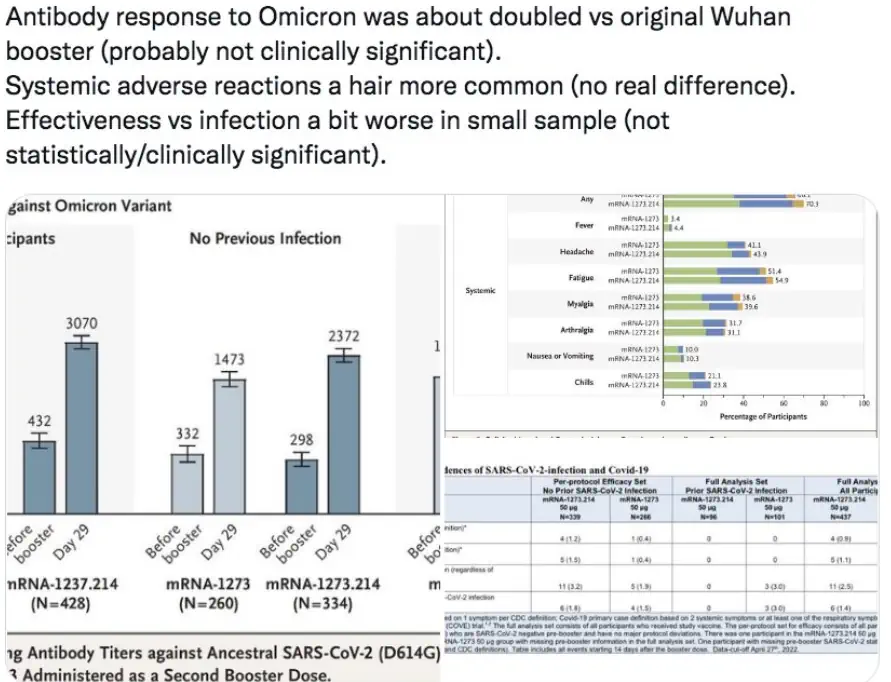
Around 3-5 weeks after a bivalent booster, meaning a fourth shot in an mRNA series, vaccinees “had similar neutralizing antibody titers as those receiving a fourth monovalent mRNA vaccine against all SARS-CoV-2 variants tested, including BA.4/BA.5,” the Columbia study found. The original boosters actually produced “slightly higher” titers against three related viruses.
“These findings may be indicative of immunological imprinting, although follow-up studies are needed to determine if the antibody responses will deviate in time, including the impact of a second bivalent booster,” according to the paper.
Two new pre-prints (Columbia U & Beth Israel Deaconess) show no additional neutralizing antibody levels from the BA4/5 booster relative to the original vax. Maybe the FDA should have asked for human data before approving?https://t.co/iknnBI47DKhttps://t.co/jsazfS7BdO
— Jay Bhattacharya (@DrJBhattacharya) October 25, 2022
“Until more data are available, regard the bivalent booster as equivalent to a booster which augments and broadens immunity, without specific anti-BA.5 properties,” Eric Topol, head of Scripps Research Translational Institute, tweeted Monday. He had previously worried about “the potential for imprinting” based on repeated jabs with the original vaccine.
“Our data show that BA.5 NAb titers were comparable following monovalent and bivalent mRNA boosters,” the Beth Israel researchers wrote. Both led to “preferential expansion” of original-strain antibody titers and “lower BA.1, BA.2, and BA.5” titers.
“Spike-specific CD8+ and CD4+ T cell responses,” which improve long-term immunity, “increased only modestly” in both boosters, which “did not substantially augment T cell responses.” The findings are consistent with those reported Oct. 6 in the New England Journal of Medicine for Moderna’a BA.1 bivalent, the paper says.
“Our findings suggest that immune imprinting by prior antigenic exposure may pose a greater challenge than currently appreciated for inducing robust immunity to SARS-CoV-2 variants,” the researchers wrote.
Robert Malone, a pioneer in mRNA vaccine development, invoked immune imprinting to explain the greater susceptibility of multi-boosted U.K. healthcare workers to Omicron reinfection in a study published this June in Science.
“All over the world, we are seeing these datasets that show that, unfortunately, the people that are dying and being hospitalized are overwhelmingly the highly vaccinated,” he told EpochTV. “It is not those that have natural immunity.”
Pfizer and Moderna left out the “numeric values of antibodies generated in people” in their press releases on the performance of their new boosters, Prasad wrote. “No one has clinical data– i.e. is there a reduction in hospitalization? Severe disease? And if so, we don’t know which people (how old) have a further reduction in severe disease or hospitalization from this vaccine.”
Even if the bivalents meaningfully increased neutralizing antibodies, that wouldn’t say much.
Answering questions from the FDA’s Vaccines and Related Biological Products Advisory Committee June 28, Pfizer Vice President for Viral Vaccines Kena Swanson admitted “there is no established correlate” between antibody levels and protection from disease. The FDA didn’t convene the committee to vote on specific Omicron boosters.
Here is a video of the exchange. pic.twitter.com/XaL33no9QR
— Technically Judicial (@techjudge) June 28, 2022
The FDA itself may be boosting vaccine hesitancy — and giving credence to the hypothesis that repeated jabs are actually worsening immune-system response to COVID — by openly discussing the possibility of more boosters.
Peter Marks, director of the agency’s Center for Biologics Evaluation and Research, told medical publisher STAT he lies awake at night “worrying that there is a certain chance that we may have to deploy another booster” in less than a year “at least for a portion of the population, perhaps older individuals.”
Even as he said he wasn’t trying to “diss the current mRNA vaccines,” including the new bivalents, Marks said they don’t cut it when it comes to long-term immunity.
“We need to look at other other types of vaccines” that might restore public confidence in vaccines, he added.
The FDA and CDC didn’t respond to queries about the divergence between their portrayal of the new boosters versus their real-world performance.
The feds require far less rigorous evidence to approve new COVID shots, whose price Pfizer said it plans to quadruple, than to simply remain neutral about low-cost COVID therapeutics such as ivermectin, falsely portrayed by the FDA as dangerous to humans despite a sterling safety profile and Nobel Prize for its inventor.
In a new study published in a Journal of the American Medical Association publication, Duke University medical researchers concluded ivermectin was not useful for outpatients with mild to moderate COVID.
They found a “median time to recovery” of 12 days in the ivermectin group, which had 10 hospitalizations or deaths, and 13 days in the placebo group, which had 9 hospitalizations or deaths.
While a comparison chart shows time to recovery was slightly better in vaccinated participants, who accounted for 47% of the study population, corresponding author Susanna Naggie told Just the News there was “no statistical difference in the treatment effect of IVM at this dose and duration for vaccinated or unvaccinated persons.”
joi220112supp3_prod_1666033255.71042Asked about the feds setting a lower evidence bar for bivalent boosters than for ivermectin, Naggie said the former were “authorized similar to new flu vaccines every year … based on the known safety profile and the benefit that has been shown, particularly for the prevention of hospitalization and death, with the prior vaccine, both primary and monovalent booster.”
The COVID vaccines’ effectiveness against hospitalization and death “have remained quite high and thus are the basis of the FDA authorizing the bivalent boosters,” she said.