Is the U.S. Military intentionally ordering the bare minimum of Pfizer’s FDA-approved COMIRNATY “vaccine”—legally mandated for all military personnel—while administering the experimental Emergency Use Authorization (EUA) Pfizer mRNA jab to service members as a general rule? It would certainly seem so based on a copy of an internal email dated May 25, 2022, titled “MTF Ordering Guidance for Pfizer Comirnaty-labeled (Tris/Sucrose?Gray Cap) vaccine,” shared by The Colonel’s Corner Telegram page.
The email, addressed to Certified Surgical Technologists (CSTs), provides specific ordering instructions for Military Treatment Facilities (MTFs) related to the “Pfizer/Comirnaty” vaccine with a gray cap. The email states, “Due to the amount of Pfizer Purple Cap vaccine currently on hand, MTFs should limit ordering the Pfizer Gray cap vaccine unless absolutely necessary by the following guidance.”
Specifying the guidance, the email continues, adding that before ordering the “Pfizer Comirnaty-labeled Gray Cap vaccine,” MTFs must first continue to use, order, and exhaust all stock of Purple Cap vaccines. However, they state the following exception:
“If the Pfizer Comirnaty-labeled (gray cap) vaccine is needed due to legal reasons (i.e., mandated), then MTFs will only order the minimum amount needed to fulfill the requirement … “

Despite government agencies repeatedly blurring the lines between Pfizer’s two experimental mRNA COVID-19 “vaccines,” the military’s MTF guidance in the email suggests that the Gray Cap is indeed the COMIRNATY “vaccine.” As previously reported by UncoverDC related to the lawsuit Church v. Biden, the legal distinction between the two jabs—the EUA administered Pfizer-BioNTech COVID-19 Vaccine and FDA Approved COMIRNATY—is significant.
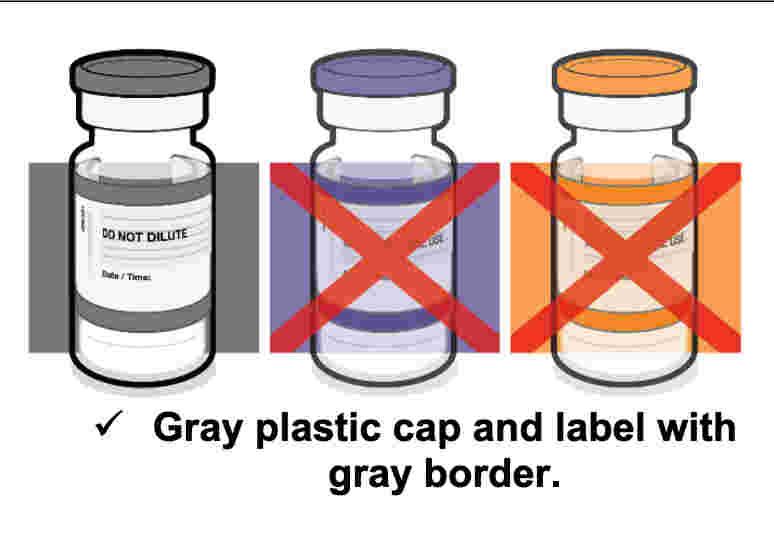
Yet, remarkably, both the Centers for Disease Control (CDC) and the Food and Drug Administration (FDA) make no apparent effort to properly differentiate between the two “vaccines,” nor do they explain which one has a Gray Cap and which one has a Purple Cap. Still, as represented in the two screenshots below, labeling data from Pfizer for the two different “vaccines” plainly indicates that its COMIRNATY jab has a Gray Cap and its Pfizer-BioNTech COVID-19 jab has a Purple Cap.
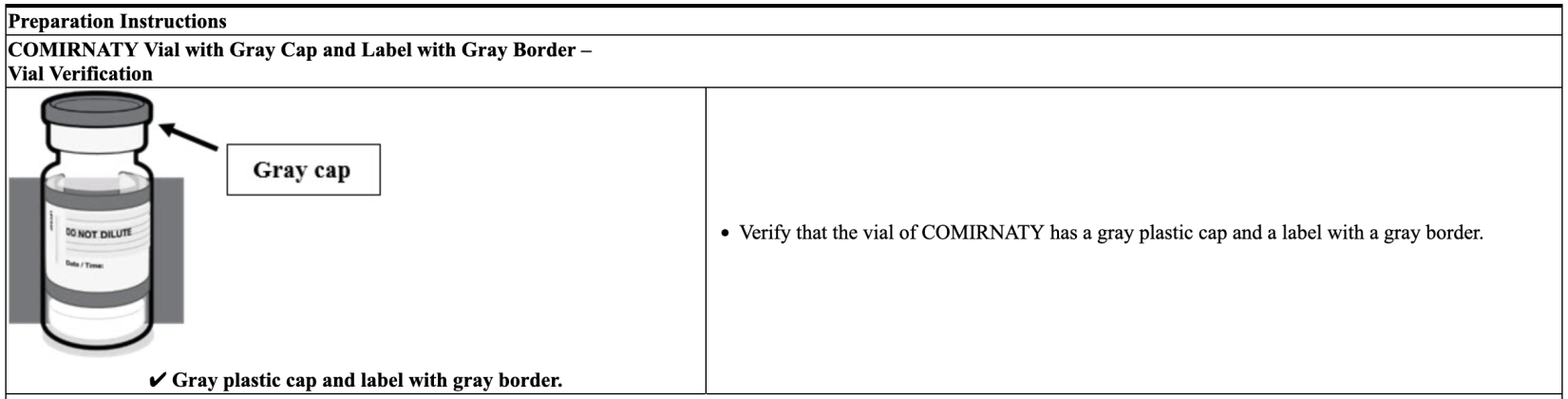
Screenshot / Pfizer Labeling for COMIRNATY, with Gray cap
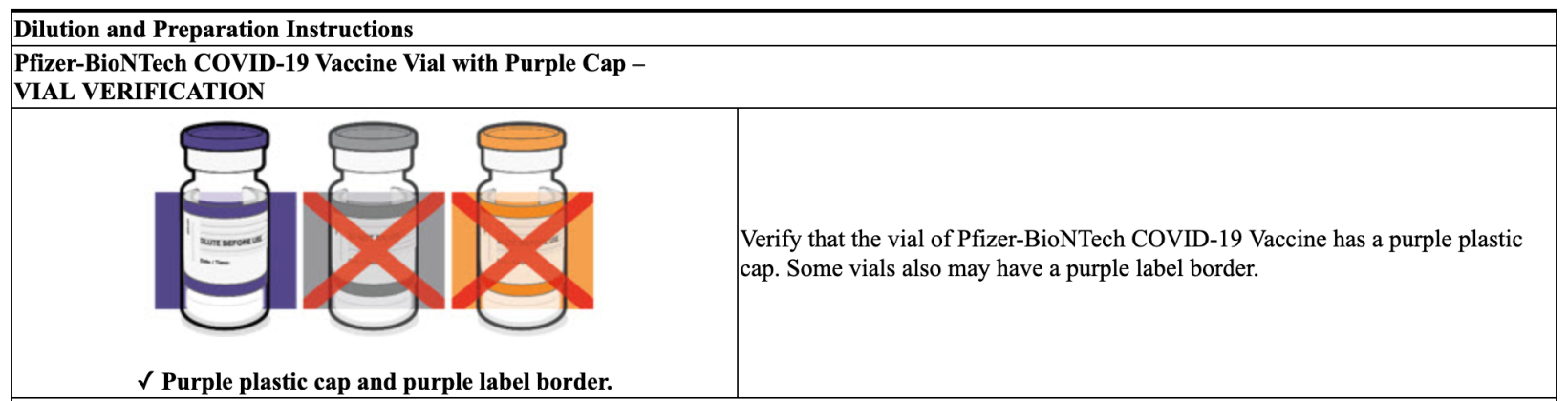
Meanwhile, even though Pfizer’s own prescribing information for the two distinct “vaccines” clarifies which product is which, it is critical to note the FDA-Approved COMIRNATY jab mandated by the U.S. Military is the only Pfizer product that has been given state and federal authority by Congress to be mandated—the EUA product has not.
Regardless of this relevant stipulation, “Fact Sheets” provided by the FDA (and referenced by the U.S. Military—remarkably, the links became inoperable as we wrote this article) for the two products are nearly identical except for detailed instructions outlining that the Gray Cap product requires no dilution and the Purple Cap product does. The FDA’s Preparation Instructions referenced by Health.mil for the Gray Cap and the Purple Cap makes no reference to COMIRNATY.
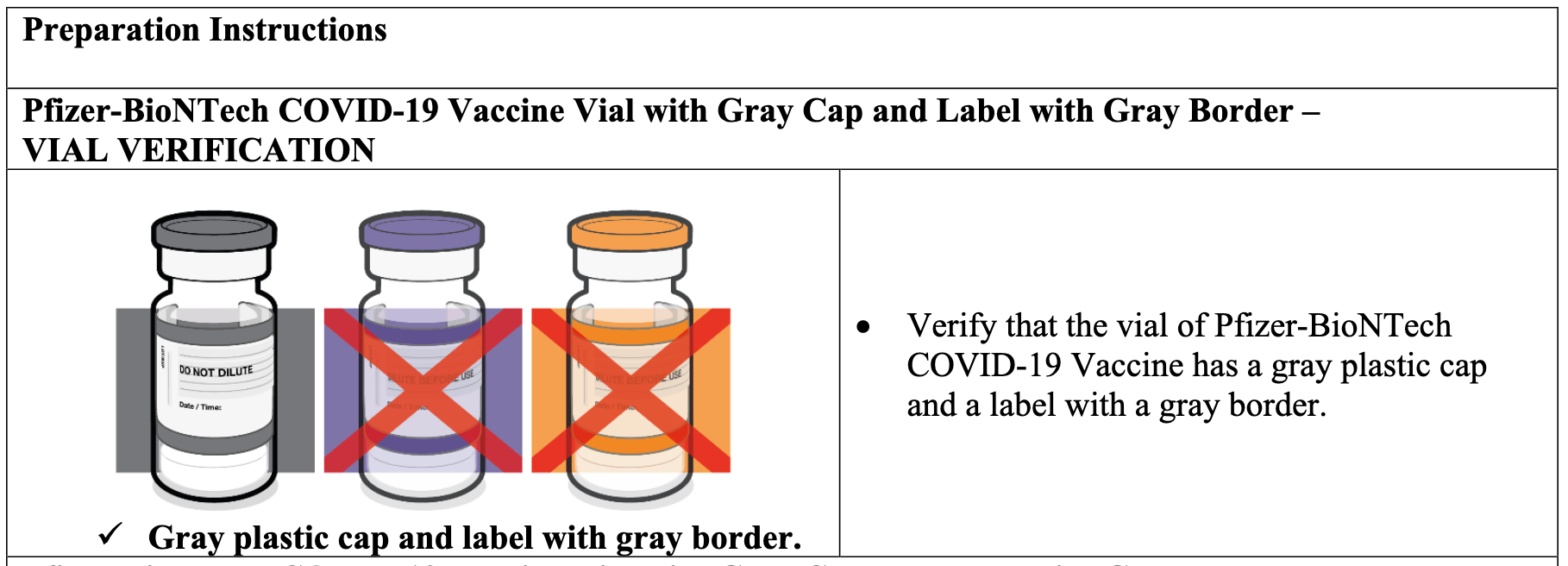
Screenshot / FDA Fact Sheet for Pfizer-BioNTech COVID-19 Vaccine with Gray Cap
Confusing the distinction between the two products further, the Health.mil website lists both the Gray and Purple Caps “vaccines” as “COMIRNATY.” The information for both products then reads, “This vaccine is authorized for emergency use in people 12 and older.”

Finally, the FDA Fact Sheet for Pfizer’s jab with the Gray Cap—which, again, Pfizer’s labeling information clearly defines as COMIRNATY—is titled, “Fact Sheet for Healthcare Providers; Emergency Use Authorization (EUA) of the Pfizer=BioNTech COVID-19 Vaccine to Prevent Coronavirus, Revised 17, May 2022.” Further establishing that the FDA regards even the Gray Cap Pfizer “vaccine” as an EUA product, page 13 of its Fact Sheet states:
“In order to mitigate the risks of using this unapproved product under EUA and to optimize the potential benefit of Pfizer-BioNTech COVID-19 Vaccine, the following items are required. Use of unapproved Pfizer-BioNTech COVID-19 Vaccine for active immunization to prevent COVID-19 under this EUA is limited …”

As reported on The Colonel’s Corner, the continued use of these experimental COVID-19 “vaccines” on our brave military members is terrible enough. Still, to do so in a manner that, by all accounts, is deceitful—leaves many with unanswered questions. Commenting on the apparent scheming revealed in the leaked May 25 email, The Colonel’s Corner remarked:
“This is the “leadership” of the military admitting they’re criminals. Knowingly plotting to poison our military members with a mandated non-FDA approved “vaccine.” Please share this everywhere.”