
An independent group of Israeli lawyers, researchers, scientists, and physicians have sent an urgent letter to the U.S. Food and Drug Administration (FDA) in advance of the Tuesday, Oct. 26, 2021, Advisory Committee Meeting to discuss Pfizer-BioNTech’s request to amend the Emergency Use Authorization (EUA) for its COVID-19 mRNA “vaccine” to include children 5 through 11 years of age. Called “Professional Ethics Front,” the nonprofit organization is deeply concerned with the integrity, quality, reliability, and legality of all Israel data associated with COVID-19 vaccination that is being used regarding the safety and efficacy of Pfizer’s “vaccine.”

The experts outline the many failures that led them to “this unfortunate, albeit inevitable” conclusion and emphasize their desire to expand further and clarify these critical missteps to the FDA. They have references and additional information for each failure described in their letter. They explain:
“We are aware that the state of Israel is perceived as ‘the world laboratory’ regarding the safety and efficacy of the Pfizer-BioNTech COVID-19 vaccine, as reflected by statements made by Dr. Albert Bourla, Dr. Anthony Fauci, and other senior figures in leading health authorities throughout the world.
It is, therefore, our understanding that the data and information coming from Israel play a crucial role in critical decision-making processes in regards to COVID-19 vaccination policies. We thus see it of utmost importance to convey a message of warning and raise our major concerns regarding potential flaws in the reliability of the Israeli data with respect to the Pfizer-BioNTech COVID-19 vaccine, as well as many significant legal and ethical violations that accompany the data collection processes.”
THIS DOCUMENT BRIEFLY OUTLINES:
“1. LACK OF A PUBLIC AND TRANSPARENT ADVERSE EVENTS REPORTING SYSTEM
2. SEVERE IMPAIRMENTS IN HEALTHCARE PROFESSIONALS’ ADVERSE EVENTS REPORTING SYSTEM
3. DATA DISTORTION
4. LEGAL AND ETHICAL VIOLATIONS IN DATA COLLECTION PROCESSES”— ROBERT W MALONE, MD (@RWMALONEMD) OCTOBER 22, 2021
The letter is signed by Dr. Sorin Schapira, MBA, Eitan Marchand, Dr. Moran Kronenberg, Dr. Sergei Bianover, Ph.D, Prof. Alon Warburg, Dr. Boaz Ilan, Prof. Eti Einhorn, Dr. Daniel Mishori, Adv. Orly Yaron, Prof. Natti Ronel, Dr. Ety Elisha, Adv. Dana Kovalskiy, Adv. Galit Polatchek, Adv. Yoram Morim, Adv. Yossi Bitton, Adv. Valentina Nelin, Dr. Ilan Makover, MD, Osnat Navon, Dr. Itsik Vorgaft, Dr. Yael Stein, MD, and Dr. Yaffa Shir-Raz.
As reported by America’s Frontline Doctors (AFLDS), who interviewed Dr. Yaffa Shir Raz in the video at the end of this article, the experts elaborate, saying:
“We believe that the significant failures underlying the Israeli database, which have been brought to our attention by numerous testimonies, impair its reliability and legality to such an extent that it should not be used for making any critical decisions regarding the COVID-19 vaccines.”
The notice expands on the dangerous failures:
“1. Lack of a Public and Transparent Adverse Events Reporting System: The first prerequisite for granting a permit for use of any new medicinal preparation is the setup of adverse events (AEs) collection systems that would allow appropriate management of risks and generation of alarm signals. All the more so when it concerns a mass vaccination campaign of a first-in-human use of an experimental preparation to the citizens of an entire country, which serves as a global model. Despite the advanced technological systems available to the Israeli HMOs, and contrary to common standards in Western countries, there exists no proper and transparent AEs reporting system in Israel, such as the U.S. VAERS system, that is accessible to the public, and thus no appropriate tracking of AEs occurring after the administration of the COVID-19 vaccine.
“Healthcare professionals or citizens in Israel, who wish to submit reports of AEs following vaccination, are unable to do so. As such, there is no possibility for either of these populations to also search through the data, rendering impossible the examination of the reported AEs by other citizens, physicians and independent researchers. Instead, there is only an online AEs reporting form available on the [Ministry of Health] MOH’s website. This form, however, was for many months not useful, since it did not allow the inclusion of personal contact information. The free text field intended to describe the AEs comprised a limited number of characters and the symptoms list available to choose from was limited as well and included only mild AEs terms.
“A petition to the Israeli Supreme Court of Justice has led the MOH to implement the above-mentioned necessary improvements to the form. Unfortunately, the modification of the form was made very late, after the majority of the adult population had already been vaccinated. Furthermore, since the report is not publicized in a transparent manner, the MOH is the only recipient and thus the sole owner of the data and the decision-making authority on the utilization and distribution of it.
“Moreover, no tracking and monitoring of even the most sensitive populations, such as pregnant women and the elderly, is taking place. For example, as part of the ‘National Senior Population Protection from the COVID-19 Program’ in Israel, a reporting system was activated in April 2020, which presented detailed reports almost daily on COVID-19 eruptions, on hospitalizations and on mortality in nursing homes. However, on December 29th, 2020, the very day the vaccination campaign commenced in nursing homes, the publication of these reports was abruptly discontinued and has never been resumed since.
“2. Severe Impairments in Healthcare Professionals’ Adverse Events Reporting System: We reveal that physicians and medical teams in Israel encounter great obstacles when attempting to report AEs following Pfizer-BioNTech COVID-19 vaccination to the MOH. We have testimonies of physicians, who attest to the complexity of filling the AEs reports to the MOH, claiming that reporting is almost impractical in the incredibly stressful working conditions of medical teams in Israel during this period. As a result of these tremendous difficulties, there is an immense underreporting of AEs by healthcare professionals in Israel, and AEs are only rarely reported in exceptional cases. The physicians’ testimonies that we have obtained also show that reported AEs are not openly publicized or made available to the healthcare professionals themselves.
“Even more disturbing is the fact that the few reports, which the Israeli MOH does publicize about the AEs observed after receiving the Pfizer-BioNTech COVID-19 vaccine, are not consistent with the testimonies of physicians regarding severe adverse events (SAEs) that they themselves have reported to the MOH. Thus, for example, in a discussion before the Advisory Committee of the FDA on September 17th, 2021, the head of the Israeli Health Services, Dr. Alroy-Preis, claimed that only one case of myocarditis was observed after the 3rd vaccine dose out of three million people who received the 3rd vaccine dose in Israel. This claim does not reconcile with research findings from all over the world, including findings from Israel, that were published in the medical literature, according to which the rate of myocarditis observed after receiving the Pfizer-BioNTech COVID-19 vaccine stands at 1:3,000-6,000. The claim of Dr. Alroy-Preis also stands in contrast to reports given by a handful of brave Israeli doctors about cases of myocarditis and other SAEs observed in close proximity to the Pfizer-BioNTech COVID-19 vaccine.
“One of these physicians, Dr. Yoav Yehezkelli, who was among the founders of the Israeli Outbreak Management Team, wrote on his Facebook page that he personally treated in his clinic a 17-year-old boy, who suffered from myocarditis several days after the 3rd vaccine dose, and he knows of two additional cases among the boy’s classmates. Dr. Yoav Yehezkelli added that he reported the myocarditis case that he treated (and additional SAEs cases) to the MOH through the online reporting system, as well as via personal reports to MOH officials, but his reports were quickly dismissed as having no link to the vaccine, without a thorough examination of the cases. Dr. Yehezkelli also mentioned that he encountered other patients in his clinic, who were hospitalized after suffering from AEs in close proximity to receiving Pfizer-BioNTech COVID-19 vaccines, and the hospital supposedly failed to report said AEs to the MOH. We have affidavits from nine other physicians, who have also treated cases of myocarditis or know of such cases, but have abandoned their attempts at reporting to the MOH having tackled immense difficulty or, alternatively, reported to the MOH and did not get any response. It is statistically improbable that a small cohort of physicians should witness these many COVID-19 vaccine injuries if Dr. Alroy-Preis’s claim was accurate.
“3. Data Distortion: Recently, two serious incidents in which data presented by the MOH was distorted have been revealed.
“The first one was the deletion of thousands of citizens’ responses to a post by the MOH. In response to a MOH post that read ‘Let’s talk about the adverse events,’ and claimed that the vaccine is completely safe and that SAEs are extremely rare, tens of thousands of responses from the public were posted, with many reporting AEs, including SAEs, which they suffered after the vaccine. But instead of examining the responses and addressing them, about half of them were deleted.
“The second event occurred about two weeks ago. Based on MOH dashboard data, an analysis conducted by members of the Israeli Public Emergency Council for the Corona Crisis (PECC) demonstrated that the Pfizer-BioNTech COVID-19 3rd vaccine dose effectiveness is much lower than that claimed in the New England Journal of Medicine study presented by Dr. Sharon Alroy-Preis to the FDA panel on September 17th, 2021. Within 24 hours of the release of the PECC analysis, the relevant dashboard data history was completely re-written. The PECC released screenshots of both the original and “rectified” data.
“4. Legal and Ethical Violations in Data Collection Processes: Not only is the data coming from Israel regarding the safety and efficacy of the Pfizer-BioNTech COVID-19 vaccine apparently unreliable, but also the collection method is controversial, and claimed to be neither legal nor ethical. The Pfizer-BioNTech COVID-19 vaccines are administered to the Israeli population without their informed consent, which is required by the GCP chapter of IHC-6 and carried out in other countries. This is a clear violation of the Nuremberg Code Rules, the Patient’s Bill of Rights, and the Israeli MOH directives for clinical trials on humans. Moreover, the Israeli citizens are under tremendous pressure to get vaccinated, almost to the point of coercion.
“Should the ‘Outbreak Management Team’ decide on a 3rd dose of the vaccine to the immunocompromised patients, it is not clear how many we can vaccinate, and it requires approval of the Helsinki Committee (medical trial approval committee) and Pfizer’s approval. We are committed to Pfizer, to vaccinate only by the vaccination regimen established by them”. This is a statement made by Prof. Hezi Levi, former CEO of the Israeli MOH, on July 5th, 2021. The evident conclusion is that the 3rd vaccine dose operation is an experiment requiring approval of the Helsinki Committee in charge of approving human medical experiments in Israel. Such an approval has never been issued. Moreover, the 3rd vaccine dose operation refers only to the immunocompromised population, and thus is even more unethical in healthy individuals, especially in young, healthy individuals, shown to be at a higher risk for myocarditis.
“We are deeply concerned with the failure of the Vaccine Safety Committee (VSC) to fulfill its designated role. The VSC is responsible in Israel for vaccine safety and the official arm designated to monitor and collect safety data. It has not issued a single position paper on its behalf or raised a single red flag to raise awareness/bring attention to SAE cases and has never gathered in full assembly. Additionally, one of the public representatives, who is a pediatrician (allergist, immunologist), never knew that he was appointed and did not attend any of the meetings, even when they did take place.”
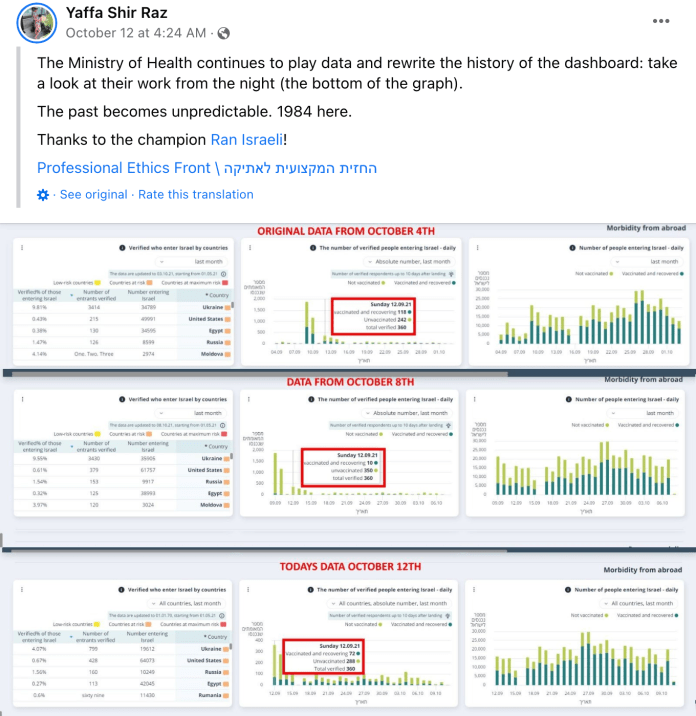
The Israeli Professional Ethics Front reminds that Israel lacks a transparent, public, and easily accessible vaccine adverse event reporting system. Indeed, the adverse events that are reported are unfortunately distorted or ignored. Recently, the group found evidence affirming that the Pfizer data was not as reliable as the Israel Minister of Health claimed. The Israeli data was presented to the FDA panel on September 17. Not even 24 hours after the release, the Israeli website holding the data was rewritten, but not before screenshots were taken of the data and its manipulation. The group maintains that the FDA and the National Institute of Health (NIH) are extremely reckless to rely on Israeli data. In its letter to the FDA, the group concludes:
“In accordance with the accepted perception established after World War II, the findings of experiments obtained in illegal and immoral ways should not be relied upon. We believe that the same rules should apply to the findings of the current experiment in Israel, since these findings were obtained through significant legal and ethical infringements. Our conclusion is further reinforced by the significant doubts about the reliability of the data reported by Israel, as detailed above, and the consequent major concern that their use might be misleading and thus disrupt the decision-making processes pertaining to the Pfizer-BioNTech COVID-19 vaccines.
“In the Book of Leviticus, it is said ‘Do not stand idly by while your neighbor’s blood is shed.’ In the spirit of those words, we implore the committee to take into consideration our urgent warnings and adopt utmost precaution when referring to the Israeli data concerning the safety and efficacy of the Pfizer-BioNTech COVID-19 vaccines.”
