 Adverse events from vaccines are common but underreported, with less than one percent reported to the Food and Drug Administration (FDA). Low reporting rates preclude or delay the identification of “problem” vaccines, potentially endangering the health of the public. New surveillance methods for drug and vaccine adverse effects are needed. Proactive, spontaneous, automated adverse event reporting embedded within electronic medical records (EMRs) and other information systems has the potential to speed the identification of problems with new vaccines and yield more careful quantification of the risks of older ones.
Adverse events from vaccines are common but underreported, with less than one percent reported to the Food and Drug Administration (FDA). Low reporting rates preclude or delay the identification of “problem” vaccines, potentially endangering the health of the public. New surveillance methods for drug and vaccine adverse effects are needed. Proactive, spontaneous, automated adverse event reporting embedded within electronic medical records (EMRs) and other information systems has the potential to speed the identification of problems with new vaccines and yield more careful quantification of the risks of older ones.
The Electronic Support for Public Health – Vaccine Adverse Event Reporting System (ESP: VAERS) project sought to create a generalizable system to facilitate detection and clinician reporting of vaccine adverse events. EMRs available from all ambulatory care encounters in a large multi-specialty practice were used. Every patient receiving a vaccine was automatically identified and for the next 30 days their health care diagnostic codes, laboratory tests, and medication prescriptions were evaluated for values suggestive of an adverse event.
The main objectives of this project were to:
- Identify required data elements and develop systems to monitor ambulatory care EMRs for adverse events following vaccine administration.
- Prepare and securely submit clinician approved, electronic reports to the national Vaccine Adverse Event Reporting System (VAERS).
- Comprehensively evaluate ESP: VAERS performance in a randomized trial and compare it with existing VAERS and Vaccine Safety Datalink data.
- Distribute documentation and application software developed and refined in 1 and 2 that are portable to other ambulatory care settings and to other EMR systems.
Preliminary data were collected from June 2006 through October 2009 on 715,000 patients. A total of 1.4 million vaccine doses (of 45 different vaccines) were given to 376,452 individuals. Of these doses, 35,570 possible reactions (2.6 percent of vaccinations) were identified. This is an average of 1.3 events per clinician per month. The team concluded that it is possible to automatically detect adverse events in defined ways, and to electronically report them to VAERS. Decision support functions can be repurposed, so that in addition to detecting reportable diseases, they can detect events that are related to vaccination, as potential vaccine adverse events.
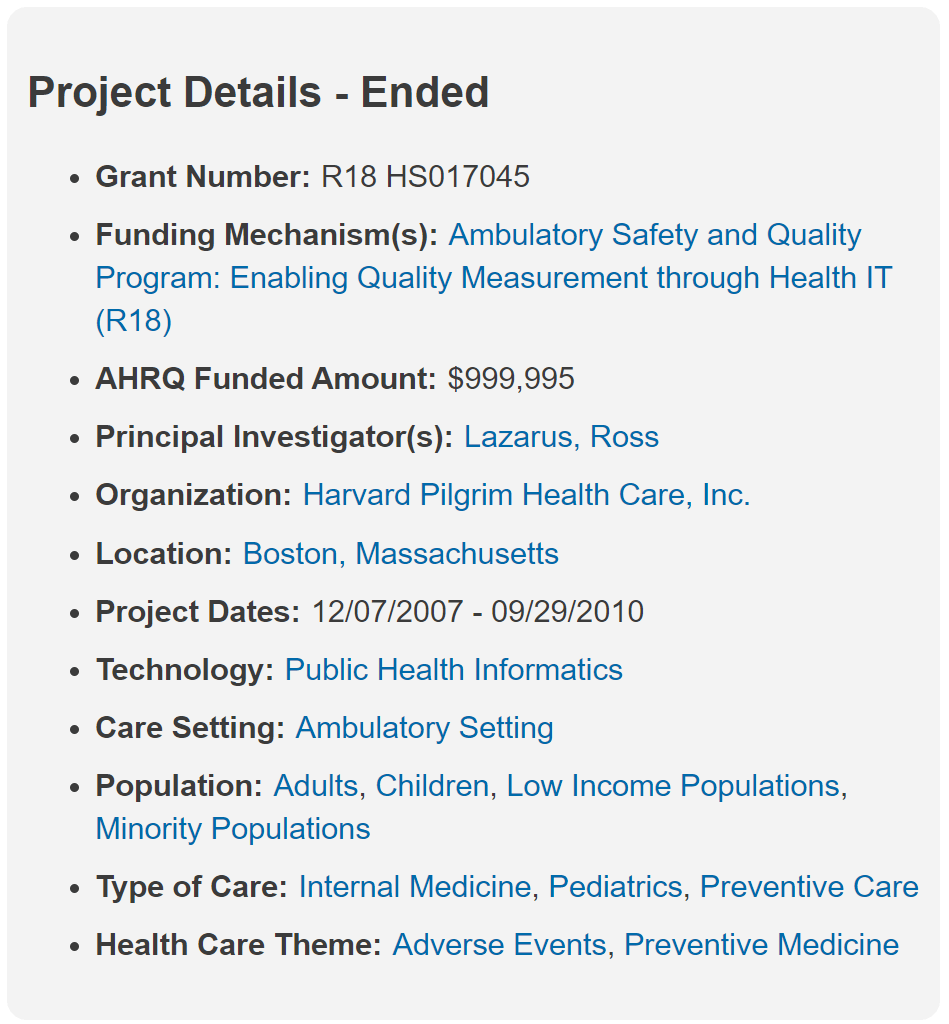
r18hs017045-lazarus-final-report-2011 Electronic Support for Public Health - Vaccine Adverse Event Reporting System (ESP_VAERS) _ Digital Healthcare Research