What is pseudouridine, why is it being injected into you, and why should you care.
 |

“If the radiance of a thousand suns were to burst at once into the sky, that would be like the splendor of the mighty one.” “Now I am become Death, the destroyer of worlds”.
J. Robert Oppenheimer, Scientific director of the Manhattan Project (quoting from the Bhagavad Gita)
Last January, Stew Peters decided to roll out the thesis that I have personal responsibility for the morbidity and mortality associated with the COVID-19 mRNA vaccines consequent to my pioneering work in developing the ideas and reduction to practice of using synthetic mRNA as a transient “gene therapy” method, with the entry level application being for vaccine purposes. This has been echoed by many angry social media detractors seeking to find someone to blame for the lies and adverse events that have been associated with these mRNA vaccines. Mindful of those critics, this Substack essay focuses on some of the differences between what was originally envisioned and the current molecules that are being injected into our bodies. The first section of the essay sets the stage by summarizing (for a general readership) how the whole idea of gene therapy was developed, and then describing how and why this lead to the idea of mRNA as a drug and as a method of generating a vaccine response. The second section gets quite technical, and provides detailed information intended for a scientific audience. The conclusion is written for a general audience.
Gene Therapy, Transhumanism, and the origins of mRNA as a drug or vaccine
The core idea captured in the original nine patents which stem from my work between 1987 and 1989 was that there are multiple key problems with the idea of permanent “gene therapy” as originally envisioned by Richard Roblin, PhD and academic Pediatrician Dr. Theodore Friedman in 1972. The modern embodiment of this concept can be found in the many writings from the WEF and others concerning “Transhumanism” and use of CRISPR/Cas9 gene editing technology. To really understand all of this requires a brief journey through the history and logic of “gene therapy”.
The January 2015 UC San Diego News center piece entitled “Friedman Recognized for Pioneering Gene Therapy Research: School of Medicine professor receives prestigious Japan Prize” nicely summarizes the underlying logic of “Gene Therapy” as envisioned by Friedman and Roblin.
“Though posed as a question, Friedmann and Roblin firmly believed the answer was yes, citing emergent thinking, new studies and growing data that suggested “good DNA” could be used to replace defective DNA in people with inherited conditions.
“In our view,” they wrote, “gene therapy may ameliorate some human genetic diseases in the future. For this reason, we believe that research directed at the development of techniques for gene therapy should continue.”
Though Friedmann said initial response to the paper was “not overwhelming,” it’s now commonly cited as a major milestone in the scientific beginnings of gene therapy research, though Friedmann said it was the Asilomar conference three years later (scientists set safety standards for recombinant DNA technology) where interest really “exploded.”
The idea of gene therapy, which quickly captured the public imagination, was fueled by its appealingly straightforward approach and what Friedmann has described as “obvious correctness”: Disarm a potentially pathogenic virus to make it benign. Stuff these viral particles with normal DNA. Then inject them into patients carrying abnormal genes, where they will deliver their therapeutic cargoes inside the defective target cells. In theory, the good DNA replaces or corrects the abnormal function of the defective genes, rendering previously impaired cells whole, normal and healthy. End of disease.”
Nice theory, what could possibly go wrong? The article continues-
“In 1968, Friedmann, working at the National Institutes of Health in Bethesda, Maryland with the late Jay Seegmiller (a founding faculty member of the School of Medicine) and others, showed that by adding foreign DNA to cultured cells from patients with Lesch-Nyhan syndrome, they could correct genetic defects that caused the rare but devastating neurological disorder. The condition was first described by William Nyhan, MD, a UC San Diego professor of pediatrics, and medical student Michael Lesch in 1964.
The feat was a powerful proof-of-concept, but subsequent efforts to advance the work to human clinical trials stalled. “We began to realize that it would be very complicated to take this idea and make it work in people,” Friedmann said, who joined the School of Medicine faculty in 1969.
In 1990, a 4-year-old girl with a congenital disease called adenoside deaminase (ADA) deficiency, which severely affects immunity and the ability to fight infections, became the first patient treated by gene therapy. White blood cells were taken from her, the normal ADA gene was inserted into them using an engineered and disabled virus and the cells re-injected. Despite initial claims of success, Friedmann said the experiment was eventually deemed a failure. The girl’s condition was not cured, and the research was found wanting.
A report commissioned by National Institutes of Health director Harold Varmus, MD, was highly critical of the entire gene therapy field and the ADA effort in particular, chiding investigators for creating a “mistaken and widespread perception of success.” Friedmann says he took the Varmus report “personally. I felt awful. It almost made me feel like I had been deceiving myself and my colleagues for more than two decades about the promise of gene therapy.” But he also knew there were “many more good people doing gene therapy research than rogues” and continued diligently and conscientiously to pursue his own research.
Nonetheless, media attention and hype about gene therapy continued to be rampant, fueled in part by over-enthusiastic opinions by some scientists. Things crashed in 1999 when an 18-year-old patient named Jesse Gelsinger, who suffered from a genetic disease of the liver, died during a clinical trial at the University of Pennsylvania. Gelsinger’s death was the first directly attributed to gene therapy. Subsequent investigations revealed numerous problems in the experimental design.”
The history of the Varmus report provides an early glimpse of the way things work at NIH and the US HHS. The Scientist appointed to head up the commission to review the science of “Gene Therapy” was none other than my graduate mentor Dr. Inder Verma, who had long been one of the leading proponents of gene therapy, and was subsequently forced to resign from the Salk Institute over a decades long record of what might most gently be called ethical lapses. But this was the scientist appointed by the overall Director of the NIH to “independently” investigate the scientific rigor and merits of the field. One hand washes the other.
What is awry with the original “gene therapy” concept? There are multiple issues, and here are a few-
1) Can you efficiently get genetic material (“polynucleotides”) into the nucleus of the majority of cells in the human body so that any genetic defects (or transhuman genetic improvements) can be made? In short, no. Human cells (and the immune system) have evolved many, many different mechanisms to resist modification by external polynucleotides. Otherwise we would already be overrun by various forms of parasitic DNA and RNA- viral and otherwise. This remains a major technical barrier, one which the “transhumanists” continue to overlook in their enthusiastic but naïve rush to play god with the human species. What are polynucleotides? Basically, the long chain polymers composed of four nucleotide bases (ATGC in the case of DNA, AUGC in the case of RNA) which carry all genetic information (that we know of) across time.
2) What about the immune system? Well, this was one of my breakthroughs way back in the late 1980s. What Ted (Friedman) originally envisioned was the simple idea that if a child had a genetic birth defect causing the body to produce a defective or not produce a critical protein (such as Lesch-Nyhan syndrome or Adenosine Deaminase Deficiency), this could be simply corrected by providing the “good gene” to complement the defect. What was not appreciated was that the immune systems of these children were “educated” during development to either recognize the “bad protein” as normal/self, or to not recognize the absent protein as normal/self. So, introduction of the “go od gene” into a person’s body would cause production of what was essentially a “foreign protein”, resulting in immunologic attack and killing of the cells which now have the ‘good gene”.
3) What happens when things go wrong and the “good gene/protein” is toxic? Well, in the current vaccine situation this is essentially the “Spike protein” problem. I get asked all the time “what can I do to eliminate the RNA vaccines from my body”, to which I have to answer – nothing. There is no technology that I know of which can eliminate these synthetic “mRNA-like” molecules from your body. The same is true for any of the many “gene therapy” methods currently being used. You just have to hope that your immune system will attack the cells that have taken up the polynucleotides and degrade (chew up) the offending large molecule that causes your cells to manufacture the toxic protein. Since virtually all current “gene therapy” methods are inefficient, and essentially deliver the genetic material randomly to a small subset of cells, there is no practical way to surgically remove the scattered, relatively rare transgenic cells. Clearance of genetically modified cells by the cellular immune system (T cells) is the only currently viable method to remove cells that have taken up the foreign genetic information (“transfection” in the case of mRNA or DNA, or “transduction” in the case of a viral vectored gene).
4) What happens if the “good gene” lands in a “bad place” in your genome? It turns out that the structure of our genome is highly evolved, and we are still relative neophytes in our current level of understanding. Despite having sequenced the human genome. The method of “insertional mutagenesis” (sticking genetic information in the form of viral DNA or other ways) has long been one of the leading methods to generate new insights into genetics – from fruit flies to frogs to fish to mice. When new DNA is inserted into chromosomes it can cause many unexpected things to happen. Like development of cancers, for example. This is why there is so much concern about the possibility that the mRNA-like polynucleotides used in the “RNA vaccines” may travel into the nucleus (where the DNA chromosomes reside) and insert or recombine with a cellular genome after reverse transcription (RNA-> DNA). Normally, with DNA-based gene therapy technologies, the FDA requires genotoxicity studies for this reason, but the FDA did not treat the “mRNA vaccine” technology as a gene therapy product.
Based on these risk considerations, the original idea behind using mRNA as a drug (for genetic therapeutic or vaccine purposes) was that mRNA is typically degraded quite rapidly once manufactured or released into a cell. mRNA stability is regulated by a number of genetic elements including the length of the “poly A tail”, but typically ranges from ½ to a couple of hours. Therefore, if natural or synthetic mRNA which is degraded by the usual enzymes is introduced into your body, it should only last for a very short time. And this has been the answer which Pfizer, BioNTech and Moderna have provided to physicians when asked “how long does the injected mRNA last after injection”.
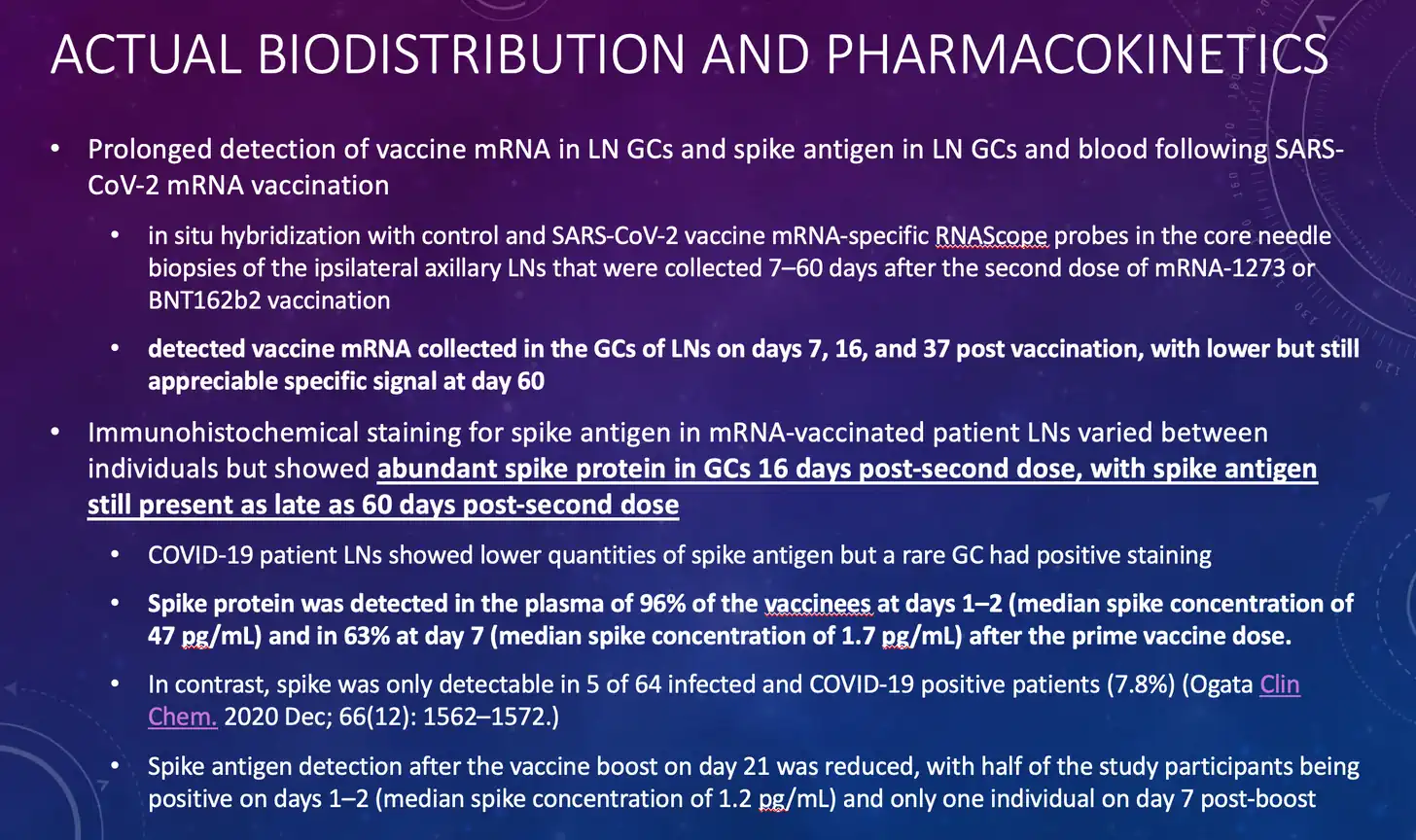
But now we know that the “mRNA” from the Pfizer/BioNTech and Moderna vaccines which incorporates the synthetic nucleotide pseudouridine can persist in lymph nodes for at least 60 days after injection. This is not natural, and this is not really mRNA. These molecules have genetic elements similar to those of natural mRNA, but they are clearly far more resistant to the enzymes which normally degrade natural mRNA, seem to be capable of producing high levels of protein for extended periods, and seem to evade normal immunologic mechanisms for eliminating cells which produce foreign proteins which are not normally observed in the body.
Key findings from this seminal work by Katharina Röltgen et al include the following:
Regarding pseudouridine and mRNA
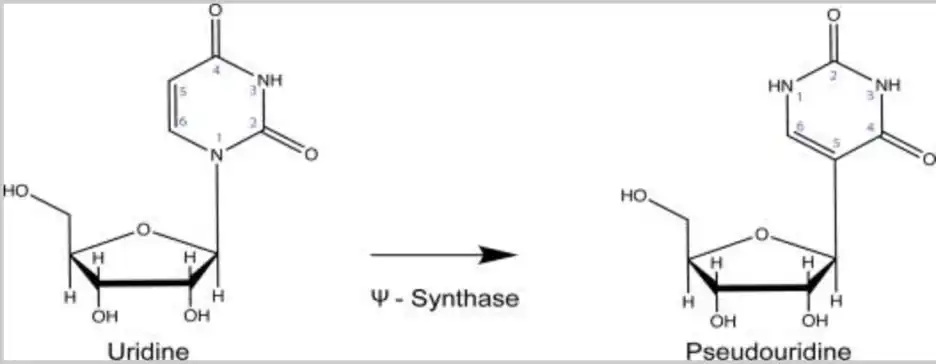
What is pseudouridine (shorthand symbol Ψ)? Pseudouridine is a modified nucleotide mRNA subunit that is prevalent in natural human mRNAs, and the biologic significance and regulation of the modification process is still being determined and understood. This modification occurs naturally in the cells of our body, in a highly regulated manner. This is in sharp contrast to the random incorporation of synthetic pseudouridine which occurs with the manufacturing process used for producing the Moderna and Pfizer/BioNTech (but not CureVac) COVID-19 “mRNA” vaccines. The “state of the art” of understanding of the biology of natural pseudouridine modifications is summarized circa late 2020 in this excellent review published in the journal Annual Review of Genetics by Erin K Borchardt et al. The open source version (not paywall protected) can be found here. Hang on, because we are about to dive into some serious immunology, molecular and cell biology.
Abstract as follows:
“Recent advances in pseudouridine detection reveal a complex pseudouridine landscape that includes messenger RNA and diverse classes of noncoding RNA in human cells. The known molecular functions of pseudouridine, which include stabilizing RNA conformations and destabilizing interactions with varied RNA-binding proteins, suggest that RNA pseudouridylation could have widespread effects on RNA metabolism and gene expression. Here, we emphasize how much remains to be learned about the RNA targets of human pseudouridine synthases, their basis for recognizing distinct RNA sequences, and the mechanisms responsible for regulated RNA pseudouridylation. We also examine the roles of noncoding RNA pseudouridylation in splicing and translation and point out the potential effects of mRNA pseudouridylation on protein production, including in the context of therapeutic mRNAs.”
A more recent (peer reviewed) publication in the journal Molecular Cell has shed light on some of the mechanisms of action associated with natural pseudouridine modification. It appears that, in the natural context, various highly regulated cellular enzymes (for example PUS1, PUS7, and RPUSD4) act on specific mRNAs and specific locations within those mRNAs while they are being made in the cell to modify the normal uridine nucleotide subunit to form pseudouridine. These modifications occur at locations associated with alternatively spliced RNA regions, are enriched near splice sites, and overlap with hundreds of binding sites for RNA-binding proteins. Latest data indicate that pre-mRNA pseudouridylation is used by human cells to regulate human gene expression via alternative pre-mRNA processing.
Relevant to the “mRNA” vaccines, the Borchardt review makes the following surprising statement, which is consistent with the Cell paper cited above which demonstrates that the synthetic “mRNA” being used for these vaccines persists in patient lymph node tissue for 60 days or longer-
“An exciting possibility is that regulated mRNA pseudouridylation controls mRNA metabolism in response to changing cellular conditions.”
That is a technically precise way of saying that incorporation of pseudouridine is one factor that controls how long an mRNA stays around in your body.
The review proceeds with the following alarming (from the context of the unregulated incorporation of Ψ into the molecules used for vaccine purposes) statement:
“The biological effects of Ψ must originate in chemical differences between U and Ψ, which primarily affect RNA backbone conformation and the stability of base pairs. Because Ψ can form stable pairs with G, C and U in addition to A, it has been proposed as a “universal” base pairing partner. Despite intensive study of the structural effects of Ψ on short, synthetic RNA oligos, it is currently impossible to predict the structural outcome of site-specific RNA pseudouridylation in longer RNAs. The systematic investigation of sequence-context effects on the stability of Ψ-containing duplexes is an important step towards accurate predictions. It will be important to determine the structural consequences of RNA pseudouridylation in cells, which is possible using improved methods to probe RNA structure in vivo.”
Furthermore,
“The effect of Ψ on the yield of functional protein depends strongly on the specific codons used. The mechanisms underlying this sequence dependence are unknown, highlighting how much remains to be understood about the translational consequences of mRNA pseudouridylation in cells.”
Finally, relevant to the immunosuppression being observed after multiple mRNA vaccine boosters (which is increasingly referred to as an acquired immunodeficiency syndrome or AIDS disease), Borchardt et al teach the following:
“Innate Immunity
Cells are equipped with innate immune sensors, including various Toll-like receptors (TLRs), retinoic acid inducible protein (RIG-I), and protein kinase R (PKR), which detect foreign nucleic acid. RNA modifications have been thought to provide a mechanism for discerning “self” RNA from non-self RNA, and indeed, incorporating RNA modifications, including pseudouridine, in foreign RNA allows for escape from innate immune detection. This makes RNA modification a powerful tool in the field of RNA therapeutics where RNAs must make it into cells without triggering an immune response, and remain stable long enough to achieve therapeutic goals. In addition, the presence of modified nucleosides in viral genomic RNA could contribute to immune evasion during infection.
TLRs Toll-Like Receptors (TLRs) are membrane-associated proteins which detect various pathogen associated molecular patterns (PAMPS) and subsequently stimulate production of proinflammatory cytokines. The RNA-sensing TLRs, TLR3, TLR7 and TLR8 reside within endosomal membranes. TLR3 recognizes dsRNA, while TLR7 and TLR8 recognize ssRNA. Upon target recognition, TLRs activate a signaling cascade that results in the expression of proinflammatory cytokines and interferon. In vitro transcribed RNA is immunostimulatory when transfected into HEK293 cells engineered to express either TLRs and inclusion of Ψ in the RNA suppressed this response (most pronounced for TLR7 and TLR8).
RIG-I Retinoic Acid Inducible Protein (RIG-I) is a cytosolic innate immune sensor responsible for detecting short stretches of dsRNA or ssRNA with either a 5′-triphosphate or 5′-disphosphate group (a feature common to various RNA viruses). Activation of RIG-I relieves its autoinhibition, releasing its CARD domains to interact with MAVS and set off a signaling cascade that ultimately results in expression of immune factors. Inclusion of Ψ in a 5′-triphosphate capped RNA abolishes activation of RIG-I, providing another mechanism for pseudouridine-mediated suppression of innate immune activation. Further, the polyU/UC region of the HCV genome is also potent activator of RIG-I and complete replacement of U with Ψ in this RNA fully abrogates downstream IFN-beta induction, despite RIG-I still binding to the modified RNA, but with reduced affinity. Durbin et al present biochemical evidence that RIG-I bound to pseudouridylated polyU/UC RNA fails to undergo the conformational changes necessary to activate downstream signaling.
PKR RNA-dependent Protein Kinase (PKR) is a cytosolic resident innate immune sensor. Upon detection of foreign RNA, PKR represses translation through phosphorylation of translation initiation factor eIF-2alpha. Molecules which activate PKR are varied, but include dsRNA formed intra- or inter-molecularly, and 5′ triphosphate groups. Inclusion of Ψ in various PKR substrates reduces PKR activation and downstream translation repression relative to unmodified RNAs. For example, a short 47-nt ssRNA potently activates PKR when synthesized with U but not with Ψ (~30-fold reduction with Ψ). Ψ also modestly reduced PKR activity when this short RNA was annealed to a complementary unmodified RNA 170. Likewise, in vitro transcribed, unmodified tRNA acted as much more potent activator of PKR than tRNAs transcribed with pseudouridine. It should be noted that it is unclear whether a fully pseudouridylated tRNA adopts canonical folding and what impact this may have on PKR recognition of this substrate. Finally, transfection of an unmodified mRNA caused a greater reduction in overall cellular protein synthesis in cell culture compared to the same mRNA fully pseudouridylated. Consistent with this result, fully pseudouridylated mRNA reduced PKR activation and subsequent phosphorylation of eIF-2alpha.”
Regarding the consequences for the use of mRNA as a drug for therapeutic or vaccine purposes, Borchardt et al conclude that
“Pseudouridine likely affects multiple facets of mRNA function, including reduced immune stimulation by several mechanisms, prolonged half-life of pseudouridine-containing RNA, as well as potentially deleterious effects of Ψ on translation fidelity and efficiency.”
Conclusion
Based on this information, it appears to me that the extensive random incorporation of pseudouridine into the synthetic mRNA-like molecules used for the Pfizer/BioNTech and Moderna SARS-CoV-2 vaccines may well account for much or all of the observed immunosuppression, DNA virus reactivation, and remarkable persistence of the synthetic “mRNA” molecules observed in lymph node biopsy tissues by Katharina Röltgen et al. Many of these adverse effects were reported by Kariko, Weissman et al in their 2008 paper “Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability” and could have been anticipated by regulatory and toxicology professionals if they had bothered to consider these findings prior to allowing emergency use authorization and widespread (global) deployment of what is truly an immature and previously untested technology. Therefore, neither the FDA, NIH, CDC, nor BioNTech (which employs Dr. Kariko as a Vice President) nor Moderna can claim true ignorance. To my eyes, what we have seen is more appropriately classified as “willful ignorance”.
In conclusion, based on these data it is my opinion that the random and uncontrolled insertion of pseudouridine into the manufactured “mRNA”-like molecules administered to so many of us creates a population of polymers which may resemble natural mRNA, but which have a variety of properties which distinguish them in a variety of aspects which are clinically relevant. These characteristics and activities may account for many of the unusual effects, unusual stability, and striking adverse events associated with this new class of vaccines. These molecules are not natural mRNA, and they do not behave like natural mRNA.
The question that most troubles and perplexes me at this point is why the biological consequences of these modifications and associated clinical adverse effects were not thoroughly investigated before widespread administration of random pseudouridine-incorporating “mRNA”-like molecules to a global population. Biology, and particularly molecular biology, is highly complex and matrix-interrelated. Change one thing over here, and it is really hard to predict what might happen over there. That is why one must do rigorously controlled non-clinical and clinical research. Once again, it appears to me that the hubris of “elite” high status scientists, physicians and governmental “public health” bureaucrats has overcome common sense, well established regulatory norms have been disregarded, and patients have unnecessarily suffered as a consequence.
When will we ever learn.