- EUA insulates vaccine manufacturers, the government, and basically everyone involved.
- Even with an approved vaccine, your legal recourse is almost non-existent.
- The Public Readiness and Emergency Preparedness Act (PREP Act) immunizes government, medical professionals, involved corporations, and vaccine manufacturers from legal prosecution.
- If you are injured, there are two vaccine compensation funds: Countermeasures Injury Compensation Program (CICP), National Vaccine Injury Compensation Program (VICP). The CICP applies to COVID-19 countermeasures (vaccines). COVID-19 falls under CICP. A comparison of the two programs is available here.
- The key problem with any of these programs is that a victim has to prove they were injured.
- To show direct causation between a Covered Countermeasure and a serious physical injury, the statute requires “compelling, reliable, valid, medical and scientific evidence.”
- The average person or law firm has virtually zero chance of ever proving this because of the extreme scientific qualifications and laboratory funding over years required to provide a clear scientific link. Consider how long it took the legal system to get victories in courts for harm caused by the tobacco industry, Monsanto with glyphosate (Roundup), and Johnson & Johnson with carcinogenic talcum powder.
Comparison of Countermeasures Injury Compensation Program (CICP) to the National Vaccine Injury Compensation Program (VICP)
| Program Categories | CICP | VICP |
|---|---|---|
| Program Authorization | Public Readiness and Emergency Preparedness Act (PREP Act) (42 U.S.C. §§ 247d-6d, 247d-6e) |
National Childhood Vaccine Injury Act of 1986, as amended (42 U.S.C. § 300aa-10, et seq.) |
| Filing Deadlines | ||
| Products Covered | Covered countermeasures are identified by the Secretary of the Department of Health and Human Services (HHS) in declarations published under the PREP Act. | Vaccines recommended for routine administration to children and/or pregnant women by the Centers for Disease Control and Prevention, subject to a Federal excise tax, and added to the Vaccine Injury Table by the Secretary of HHS.Covered Vaccines |
| Process for Adding Covered Vaccines/ Countermeasures | Covered countermeasures are identified by the Secretary of HHS in declarations published under the PREP Act. | For a category of vaccines to be covered by the VICP, the category of vaccines must be recommended for routine administration to children and/or pregnant women by the Centers for Disease Control and Prevention, subject to an excise tax by federal law, and added to the Vaccine Injury Table by the Secretary of Health and Human Services. This has not been done for any U.S. licensed COVID-19 vaccines, which have not been developed to date. |
| Type of Injury Covered |
|
|
| Benefits Available | Types of Benefits | Types of Compensation |
| Payment of Legal Fees and Costs | Attorneys’ fees and costs are not paid by the program. | Attorneys’ fees and costs may be available if certain requirements are met (petition filed in good faith and on a reasonable basis) |
| Persons who can file Requests/ Petitions | Types of Eligible Requesters | Who Can File a Petition? |
| Process for Filing a Request/Petition | File the Request Form and documentation with the Secretary of HHS. | File petition and documentation with the U.S. Court of Federal Claims and the Secretary of HHS. |
| Process for Resolving Requests/ Petitions | Administrative Process | Judicial Process |
| Covered Injury Determinations | HHS makes decision. | Special Masters (or judges) of U.S. of Court of Federal Claims make decision.Criteria to be Found Eligible to Receive Compensation |
| Appeal Rights | One step administrative reconsideration possible. No judicial appeal permitted. | Judicial appeal by either party to higher courts possible. |
| Program Funding | Appropriated Funds | Vaccine Injury Compensation Trust Fund funded through excise taxes on covered vaccines. Congress annually appropriates funding from the Trust Fund to the Health Resources and Services Administration (HRSA), Department of Justice, and U.S. Court of Federal Claims to pay their expenses to administer the VICP and to HRSA to compensate VICP claims. |
Vaccine Compensation
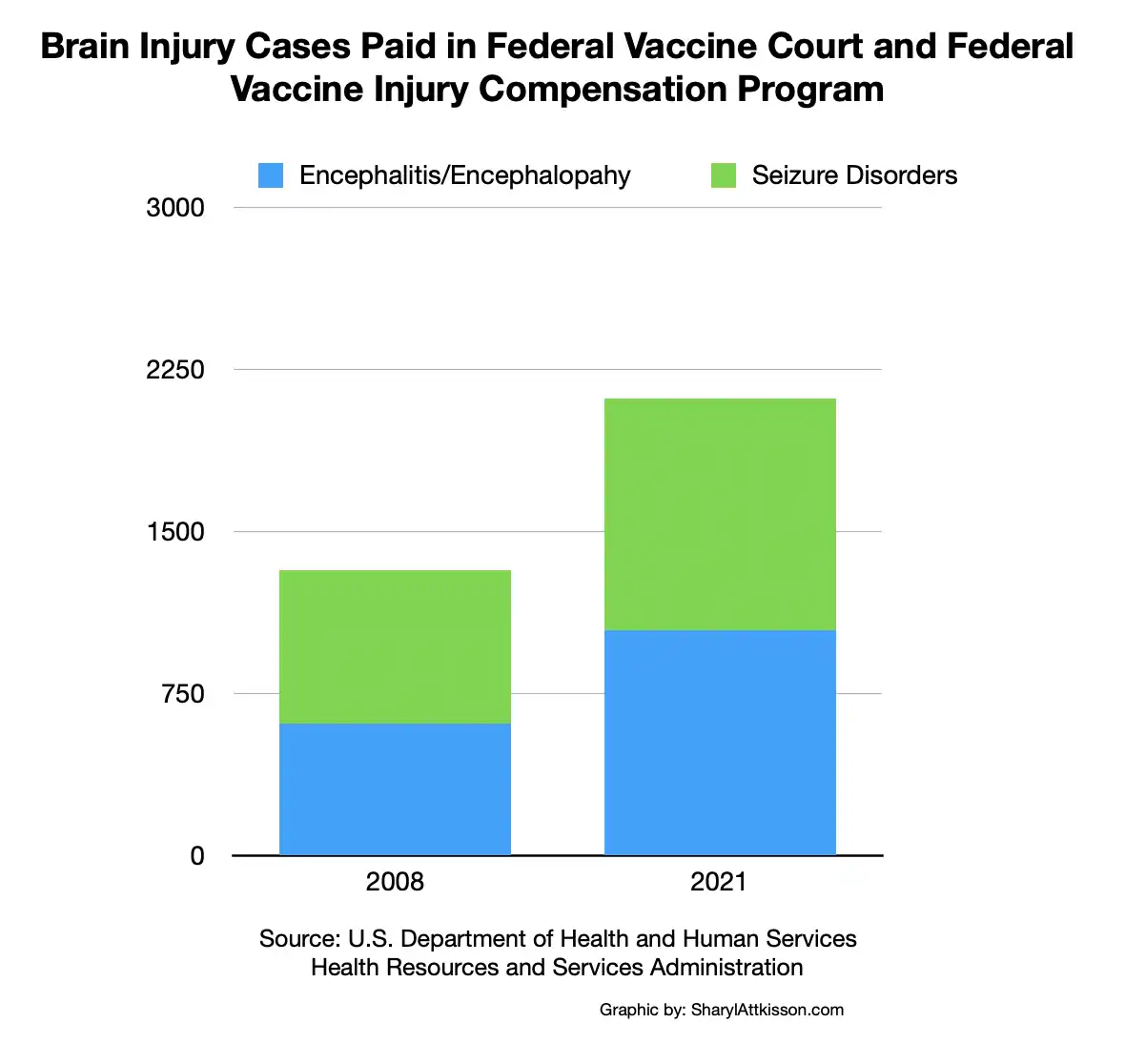
- Updated govt. numbers on brain injury cases paid in federal vaccine court
- Through July 19, 2021, the government has paid 1,045 cases of Encephalitis/Encephalopathy and 1,068 cases of Seizure Disorder after vaccination.
- To date, more than $4.6 billion has been paid to vaccine-injured children and adults through the federal government’s Vaccine Injury Compensation Program (VICP).