Andrew Bryant, MSc,1,* Theresa A. Lawrie, MBBCh, PhD,2 Therese Dowswell, PhD,2 Edmund J. Fordham, PhD,2 Scott Mitchell, MBChB, MRCS,3 Sarah R. Hill, PhD,1 and Tony C. Tham, MD, FRCP4
Background:
Repurposed medicines may have a role against the SARS-CoV-2 virus. The antiparasitic ivermectin, with antiviral and anti-inflammatory properties, has now been tested in numerous clinical trials.
Areas of uncertainty:
We assessed the efficacy of ivermectin treatment in reducing mortality, in secondary outcomes, and in chemoprophylaxis, among people with, or at high risk of, COVID-19 infection.
Data sources:
We searched bibliographic databases up to April 25, 2021. Two review authors sifted for studies, extracted data, and assessed risk of bias. Meta-analyses were conducted and certainty of the evidence was assessed using the GRADE approach and additionally in trial sequential analyses for mortality. Twenty-four randomized controlled trials involving 3406 participants met review inclusion.
Therapeutic Advances:
Meta-analysis of 15 trials found that ivermectin reduced risk of death compared with no ivermectin (average risk ratio 0.38, 95% confidence interval 0.19–0.73; n = 2438; I2 = 49%; moderate-certainty evidence). This result was confirmed in a trial sequential analysis using the same DerSimonian–Laird method that underpinned the unadjusted analysis. This was also robust against a trial sequential analysis using the Biggerstaff–Tweedie method. Low-certainty evidence found that ivermectin prophylaxis reduced COVID-19 infection by an average 86% (95% confidence interval 79%–91%). Secondary outcomes provided less certain evidence. Low-certainty evidence suggested that there may be no benefit with ivermectin for “need for mechanical ventilation,” whereas effect estimates for “improvement” and “deterioration” clearly favored ivermectin use. Severe adverse events were rare among treatment trials and evidence of no difference was assessed as low certainty. Evidence on other secondary outcomes was very low certainty.
Conclusions:
Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.
INTRODUCTION
To date, very few treatments have been demonstrated to reduce the burden of morbidity and mortality from COVID-19. Although corticosteroids have been proven to reduce mortality in severe disease,1 there has been little convincing evidence on interventions that may prevent disease, reduce hospitalizations, and reduce the numbers of people progressing to critical disease and death.
Ivermectin is a well-known medicine that is approved as an antiparasitic by the World Health Organization and the US Food and Drug Administration. It is widely used in low- and middle-income countries (LMICs) to treat worm infections.2,3 Also used for the treatment of scabies and lice, it is one of the World Health Organization’s Essential Medicines.4 With total doses of ivermectin distributed apparently equaling one-third of the present world population,5 ivermectin at the usual doses (0.2–0.4 mg/kg) is considered extremely safe for use in humans.6,7 In addition to its antiparasitic activity, it has been noted to have antiviral and anti-inflammatory properties, leading to an increasing list of therapeutic indications.8
Since the start of the SARS-CoV-2 pandemic, both observational and randomized studies have evaluated ivermectin as a treatment for, and as prophylaxis against, COVID-19 infection. A review by the Front Line COVID-19 Critical Care Alliance summarized findings from 27 studies on the effects of ivermectin for the prevention and treatment of COVID-19 infection, concluding that ivermectin “demonstrates a strong signal of therapeutic efficacy” against COVID-19.9 Another recent review found that ivermectin reduced deaths by 75%.10 Despite these findings, the National Institutes of Health in the United States recently stated that “there are insufficient data to recommend either for or against the use of ivermectin for the treatment of COVID-19,”11 and the World Health Organization recommends against its use outside of clinical trials.12
Ivermectin has exhibited antiviral activity against a wide range of RNA and some DNA viruses, for example, Zika, dengue, yellow fever, and others.13 Caly et al14 demonstrated specific action against SARS-CoV-2 in vitro with a suggested host-directed mechanism of action being the blocking of the nuclear import of viral proteins14,15 that suppress normal immune responses. However, the necessary cell culture EC50 may not be achievable in vivo.16 Other conjectured mechanisms include inhibition of SARS-CoV-2 3CLPro activity17,18 (a protease essential for viral replication), a variety of anti-inflammatory effects,19 and competitive binding of ivermectin with the viral S protein as shown in multiple in silico studies.20 The latter would inhibit viral binding to ACE-2 receptors suppressing infection. Hemagglutination via viral binding to sialic acid receptors on erythrocytes is a recently proposed pathologic mechanism21 that would be similarly disrupted. Both host-directed and virus-directed mechanisms have thus been proposed, the clinical mechanism may be multimodal, possibly dependent on disease stage, and a comprehensive review of mechanisms of action is warranted.
Developing new medications can take years; therefore, identifying existing drugs that can be repurposed against COVID-19 that already have an established safety profile through decades of use could play a critical role in suppressing or even ending the SARS-CoV-2 pandemic. Using repurposed medications may be especially important because it could take months, possibly years, for much of the world’s population to get vaccinated, particularly among LMIC populations.
Currently, ivermectin is commercially available and affordable in many countries globally.6 A 2018 application for ivermectin use for scabies gives a direct cost of $2.90 for 100 12-mg tablets.22 A recent estimate from Bangladesh23 reports a cost of US$0.60—US$1.80 for a 5-day course of ivermectin. For these reasons, the exploration of ivermectin’s potential effectiveness against SARS-CoV-2 may be of particular importance for settings with limited resources.24 If demonstrated to be effective as a treatment for COVID-19, the cost-effectiveness of ivermectin should be considered against existing treatments and prophylaxes.
The aim of this review was to assess the efficacy of ivermectin treatment among people with COVID-19 infection and as a prophylaxis among people at higher risk of COVID-19 infection. In addition, we aimed to prepare a brief economic commentary (BEC) of ivermectin as treatment and as prophylaxis for COVID-19.25
METHODS
The conduct of this review was guided by a protocol that was initially written using Cochrane’s rapid review template and subsequently expanded to a full protocol for a comprehensive review.26
Search strategy and selection criteria
Two reviewers independently searched the electronic databases of Medline, Embase, CENTRAL, Cochrane COVID-19 Study Register, and Chinese databases for randomized controlled trials (RCTs) up to April 25, 2021 (see Appendix 1–3, Supplemental digital content 1, http://links.lww.com/AJT/A95); current guidance25 for the BEC was followed for a supplementary search of economic evaluations. There were no language restrictions, and translations were planned to be performed when necessary.
We searched the reference list of included studies, and of two other 2021 literature reviews on ivermectin,9 as well as the recent WHO report, which included analyses of ivermectin.12 We contacted experts in the field (Drs. Andrew Hill, Pierre Kory, and Paul Marik) for information on new and emerging trial data. In addition, all trials registered on clinical trial registries were checked, and trialists of 39 ongoing trials or unclassified studies were contacted to request information on trial status and data where available. Many preprint publications and unpublished articles were identified from the preprint servers MedRχiv and Research Square, and the International Clinical Trials Registry Platform. This is a rapidly expanding evidence base, so the number of trials are increasing quickly. Reasons for exclusion were recorded for all studies excluded after full-text review.
Data analysis
We extracted information or data on study design (including methods, location, sites, funding, study author declaration of interests, and inclusion/exclusion criteria), setting, participant characteristics (disease severity, age, gender, comorbidities, smoking, and occupational risk), and intervention and comparator characteristics (dose and frequency of ivermectin/comparator). The primary outcome for the intervention component of the review included death from any cause and presence of COVID-19 infection (as defined by investigators) for ivermectin prophylaxis. Secondary outcomes included time to polymerase chain reaction (PCR) negativity, clinical recovery, length of hospital stay, admission to hospital (for outpatient treatment), admission to ICU or requiring mechanical ventilation, duration of mechanical ventilation, and severe or serious adverse events, as well as post hoc assessments of improvement and deterioration. All of these data were extracted as measured and reported by investigators. Numerical data for outcomes of interest were extracted according to intention to treat.
If there was a conflict between data reported across multiple sources for a single study (eg, between a published article and a trial registry record), we contacted the authors for clarification. Assessments were conducted by 2 reviewers (T.L., T.D., A.B., or G.G.) using the Cochrane RCT risk-of-bias tool.27 Discrepancies were resolved by discussion.
Continuous outcomes were measured as the mean difference and 95% confidence intervalss (CI), and dichotomous outcomes as risk ratio (RR) and 95% CI.
We did not impute missing data for any of the outcomes. Authors were contacted for missing outcome data and for clarification on study methods, where possible, and for trial status for ongoing trials.
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the I2 statistic (I2 ≥60% was considered substantial heterogeneity),28 by a formal statistical test to indicate statistically significant heterogeneity,29 and, where possible, by subgroup analyses (see below). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported. We assessed reporting biases using funnel plots if more than 10 studies contributed to a meta-analysis.
We meta-analyzed data using the random effects model (DerSimonian and Laird method)30 using RevMan 5.4.1 software.27,31 The results used the inverse variance method for weighting.27 Some sensitivity analyses used other methods that are outlined below and some calculations were performed in R32 through an interface33 to the netmeta package.34 Where possible, we performed subgroup analyses grouping trials by disease severity, inpatients versus outpatients, and single dose versus multiple doses. We performed sensitivity analyses by excluding studies at high risk of bias. We conducted further post hoc sensitivity analyses using alternative methods to test the robustness of results in the presence of zero events in both arms in a number of trials35 and estimated odds ratios [and additionally RR for the Mantel–Haenszel (MH) method] using a fixed effects model. The models incorporate evidence from single-zero studies without having to resort to continuity corrections. However, double-zero studies are excluded from the analysis; so, the risk difference was also assessed using the MH method as this approach can adequately incorporate trials with double-zero events. This method can also use a random-effects component. A “treatment-arm” continuity correction was used, where the values 0.01, 0.1, and 0.25 were added where trials reported zero events in both arms. It has been shown that a nonfixed continuity correction is preferable to the usual 0.5.35 Other methods are available but were not considered due to difficulty in interpretation, sensitivity of assumptions, or the fact they are rarely used in practice.36–40
Trial sequential analysis
When a meta-analysis is subjected to repeated statistical evaluation, there is an exaggerated risk that “naive” point estimates and confidence intervals will yield spurious inferences. In a meta-analysis, it is important to minimize the risk of making a false-positive or false-negative conclusion. There is a trade-off between the risk of observing a false-positive result (type I error) and the risk of observing a false-negative result (type II error). Conventional meta-analysis methods (eg, in RevMan) also do not take into account the amount of available evidence. Therefore, we examined the reliability and conclusiveness of the available evidence using trial sequential analyses (TSA).41–43 The DerSimonian–Laird (DL) method was used because this is most often used in meta-analytic practice and was also used in the primary meta-analysis.30
The TSA was used to calculate the required information size (IS) to demonstrate or reject a relative reduction in the risk (RRR) of death in the ivermectin group, as found in the primary meta-analysis. We assumed the estimated event proportion in the control group from the meta-analysis because this is the best and most representative available estimate. Recommended type I and II error rates of 5% and 10% were used, respectively (power of 90%),43 powering the result on the effect observed in the primary meta-analyses. We did not identify any large COVID-19 trials powered on all-cause mortality, so powering on some external meaningful difference was not possible. Any small RRR is meaningful in this context, given the scale of the pandemic, but the required IS would be unfeasibly high for this analysis if powered on a small difference. The only reliable data on ivermectin in its repurposed role for treatment against COVID-19 will be from the primary meta-analysis. Therefore, assuming it does not widely deviate from other published systematic reviews, a pragmatic decision was therefore made to power on the pooled meta-analysis effect estimate for all-cause mortality a priori. This is more reflective of a true meaningful difference. We used a model variance-based estimate to correct for heterogeneity. A continuity correction of 0.01 was used in trials that reported zero events in one or both arms. The required IS is the sample size required for a reliable and conclusive meta-analysis and is at least as large as that needed in a single powered RCT. The heterogeneity corrected required IS was used to construct sequential monitoring boundaries based on the O’Brien–Fleming type alpha-spending function for the cumulative z-scores (corresponding to the cumulative meta-analysis),43 analogous to interim monitoring in an RCT, to determine when sufficient evidence had been accrued. These monitoring boundaries are relatively insensitive to the number of repeated significance tests. They can be used to further contextualize the original meta-analysis and enhance our certainty around its conclusions. We used a two-sided test, so also considered futility boundaries (to test for no statistically significant difference) and the possibility that ivermectin could harm. Sensitivity analyses were performed excluding the trial of Fonseca,44 which was a cause of substantial heterogeneity (but retained in the core analysis because it was at low risk of bias). Its removal dramatically reduced I2 and D2 (diversity) estimates, thus reducing the model variance-based estimate to correct for heterogeneity. Two further sensitivity analyses were performed using 2 alternative random effect models, namely the Biggerstaff–Tweedie (BT) and Sidik–Jonkman (SJ) methods.43
All outcomes have been assessed independently by 2 review authors (T.D. and A.B.) using the GRADE approach,45 which ranks the quality and certainty of the evidence. The results of the TSAs will also form part of the judgment for the primary all-cause mortality outcome. The results are presented in a summary of findings table. Any differences in judgments were resolved by discussion with the wider group. We used Cochrane Effective Practice and Organisation of Care guidance to interpret the evidence.46
RESULTS
Search results and risk-of-bias assessment
The combined and preliminary deduplicated total was n = 583. We also identified 11 records from other sources (reference lists, etc). See PRISMA flow diagram for inclusion and exclusion details of these references (Figure (Figure11).
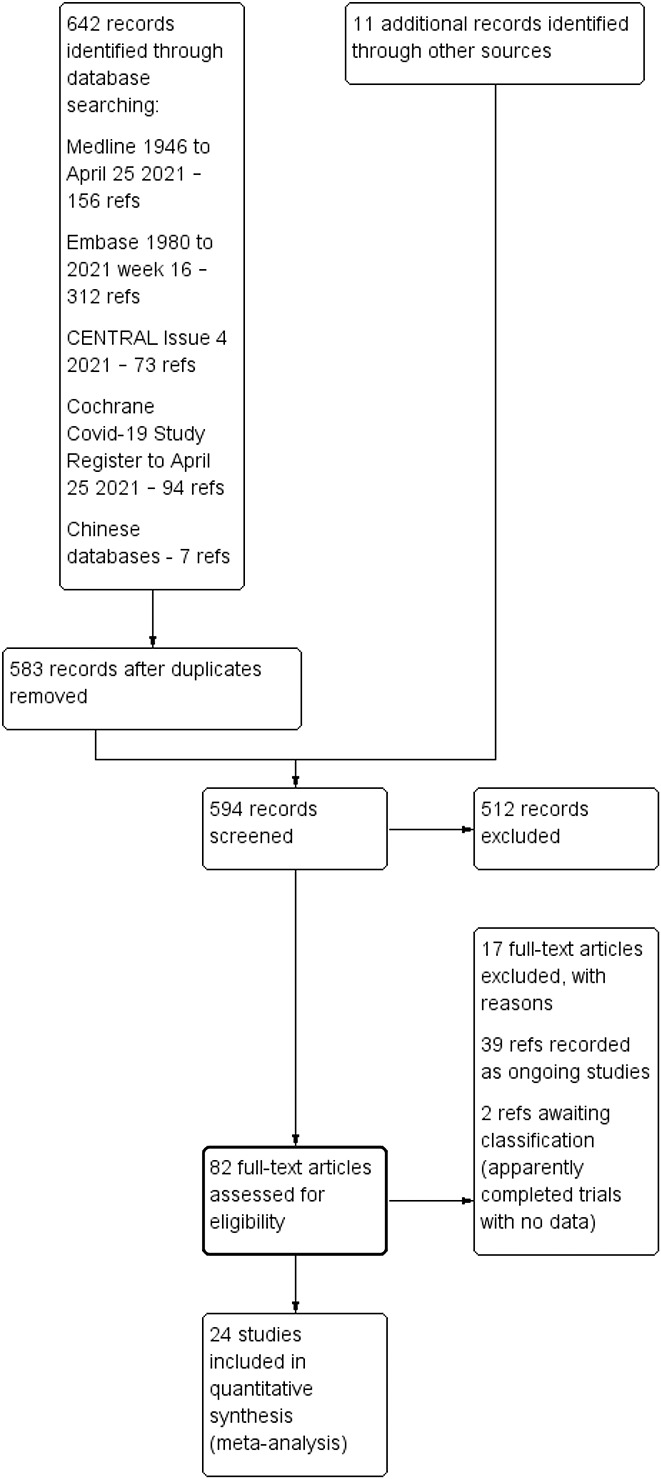
Study flow diagram from search on 25 April 2021.
The supplementary search for the BEC identified 17 studies, of which 4 were retrieved in full. No full trial- or model-based economic evaluations (cost–utility analyses, cost–effectiveness analyses, or cost–benefit analyses) were identified.
Twenty-one trials in treatment and 2 trials in prophylaxis of COVID-19 met review inclusion. One further study47 reported separate treatment and prophylaxis components; we label this study “Elgazzar” under both questions. In effect, there were 22 trials in treatment and 3 in prophylaxis. All of these contributed data to at least one review outcome and meta-analysis. Fifteen trials contributed data for the primary outcome for ivermectin treatment (death); 3 studies reported the primary outcome for prophylaxis (COVID-19 infection). Characteristics of included studies are given in Table Table1.1. Seventeen studies47–63 were excluded as they were not RCTs and we identified 39 ongoing studies64–102 and 2 studies103,104 are awaiting classification.
Table 1.
Summary of study characteristics.
| Study ID | Country | Design | Funding | Participants | Sample size | Ivermectin dose and frequency* | Comparator | Origin of data | Main outcomes reported |
| COVID-19 treatment studies | |||||||||
| Ahmed 202023 | Bangladesh | Double-blind | BPL(Pharma); Bangladesh, Canada, Sweden, and UK govt | Mild to moderate COVID (inpatients) | 72 | 12 mg × 1 day or × 5 days (3 study arms)* | Placebo | Published in PR journal; emailed/responded with data | Time to viral clearance (PCR –ve), remission of fever and cough within 7 days, duration of hospitalization, mortality, failing to maintain sats >93%, adverse events, PCR –ve at 7 and 14 days |
| Babalola 2020105 | Nigeria | Double-blind | Self-funded | Asymptomatic, mild or moderate COVID (45 inpatients and 17 outpatients) | 62 | 6 mg every 84 hrs × 2 wks (arm 1) or 12 mg every 84 hrs × 2 wks (arm 2) | Ritonavir/lopinavir | MedRxiv preprint: emailed/responded with data. Paper accepted for publication | Time to PCR –ve, laboratory parameters (platelets, lymphocytes, clotting time), clinical symptom parameters |
| Bukhari 2021135 | Pakistan | Open-label | None reported | Mild to moderate COVID (inpatients) | 100 | 12 mg × 1 dose | SOC | MedRxiv preprint | Viral clearance, any adverse side effects, mechanical ventilation |
| Chaccour 202024 | Spain | Double-blind | Idapharma, ISGlobal, and the University of Navarra | Mild COVID (outpatients) | 24 | 0.4 mg/kg × 1 dose | Placebo | Published in PR journal | PCR +ve at day 7, proportion symptomatic at day 4,7,14,21, progression, death, adverse events |
| Chachar 2020112 | Pakistan | Open-label | Self-funded | Mild COVID (outpatients) | 50 | 12 mg at 0, 12, and 24 hours (3 doses) | SOC | Published in PR journal | Symptomatic at day 7 |
| Chowdhury 2020136 | Bangladesh | Quasi-RCT | None reported | Outpatients with a +ve PCR (approx. 78% symptomatic) | 116 | 0.2 mg/kg x1 dose* | HCQ 400 mg 1st day then 200 mg BID × 9 days + AZM 500 mg daily × 5 days | Research square preprint | Time to –ve PCR test; period to symptomatic recovery; adverse events |
| Elgazzar 202047 | Egypt | RCT | None reported | Mild to severe COVID (inpatients) | 200 | 0.4 mg/kg daily × 4 days | HCQ 400 mg BID × 1 day then 200 mg BID × 9 days | Research square preprint: emailed/responded with data | Improved, progressed, died. Also measured CRP, D-dimers, HB, lymphocyte, serum ferritin after one week of treatment |
| Fonseca 202144 | Brazil | Double-blind | Institution-funded | Moderate to severe (inpatients) | 167 | 14 mg daily × 3 days (plus placebos × 2 additional days) | HCQ—400 mg BID on day 0 then daily × 4 days; CQ -450 mg BID day 0 then daily × 4 days | Prepublication data/manuscript in progress obtained via email | Death, invasive mechanical ventilation |
| Gonzalez 2021137 | Mexico | Double-blind | Institution-funded | Moderate to severe (inpatients) | 108 | 12 mg × 1 dose | Placebo | MedRxiv preprint | Length of hospital stay, invasive mechanical ventilation, death, time to negative PCR |
| Hashim 2020138 | Iran | Quasi-RCT | None reported | Mild to critical (inpatients) | 140 | 0.2 mg/kg × 2 days* Some had a 3rd dose a week later |
SOC | MedRxiv preprint | Death, mean time to recovery, disease progression (deterioration) |
| Krolewiecki 2020106 | Argentina | Open-label | None reported | Mild to moderate (inpatients) | 45 | 0.6 mg/kg/d × 5 days | Placebo | Research Gate and SSRN preprints | Viral load reduction in respiratory secretions day 5, IVM concentrations in plasma, severe adverse events |
| Lopez-Medina 202185 | Columbia | Double-blind | Institution-funded | Mild (outpatients) | 476 | 0.3 mg/kg elixir × 5 days | Placebo | Published in a PR journal | Resolution of symptoms within 21 days, deterioration, clinical condition, hospitalization, adverse events |
| Mahmud 2020107 | Bangladesh | Double-blind | None reported | Mild to moderate COVID (inpatients) | 363 | 12 mg × 1 dose* | Placebo + SOC | Data published on clinical trial registry and clarification obtained via email | Improvement, deterioration, late clinical recovery, persistent PCR test +ve |
| Mohan 2021110 | India | Double-blind | Institution-funded | Mild to moderate | 152 | 12 mg or 24 mg elixir × 1 dose | Placebo | MedRxiv preprint research | Conversion of RT-PCR to negative result, decline of viral load at day 5 from enrollment |
| Niaee 2020108 | Iran | Double-blind | Institution-funded | Mild to severe COVID | 180 | 0.2 mg/kg × 1 and 3 other dosing options) ∼ 14 mg tablet† | Placebo | Research Square preprint | Deaths, length of stay, biochemical parameters |
| Okumus 2021115 | Turkey | Quasi-RCT | None reported | Severe COVID | 66 | 0.2 mg/kg × 5 days | SOC | Prepublication data/manuscript in progress obtained via email | Clinical improvement, deterioration, death, SOFA scores |
| Petkov 2021139 | Bulgaria | Double-blind | Pharma-funded | Mild to moderate COVID | 100 | 0.4 mg/kg × 3 days | Placebo | Prepublication data obtained from another source | Rate of conversion to PCR negative |
| Podder 2020140 | Bangladesh | Open-label | Self-funded | Mild to moderate (outpatients) | 62 | 0.2 mg/kg × 1 dose | SOC | Published in PR journal | Duration of symptoms, recovery time to symptom free from enrollment, recovery time to symptom free from symptom onset, repeat PCR result on day 10 |
| Raad 2021113 | Lebanon | Double-blind | Self-funded | Asymptomatic outpatients | 100 | 9 mg PO if 45 kg–64 kg, 12 mg PO if 65 kg–84 kg and 0.15 mg/kg if body weight ≥85 kg | Placebo | Prepublication data/manuscript in progress obtained via email | Viral load reduction, hospitalization, adverse effects |
| Ravikirti 2021109 | India | Double-blind | Self-funded | Mild to moderate COVID (inpatients) | 112 | 12 mg × 2 days + SOC | Placebo + SOC | Published in PR journal | A negative RT-PCR report on day 6, symptomatic on day 6, discharge by day 10, admission to ICU, need for invasive mechanical ventilation, mortality |
| Rezai 2020111 | Iran | Double-blind | None reported | Mild to moderate (inpatient) | 60 | 0.2 mg/kg × 1 dose | SOC | Prepublication data obtained from another source | Clinical symptoms, respiratory rate and O2 saturation |
| Schwartz 2021114,141 | Israel | Double-blind | None reported | Mild to moderate (outpatients) | 94 | 0.15–0.3 mg/kg × 3 days | Placebo | Prepublication data obtained from another source | Viral clearance at day 4, 6, 8 and 10), hospitalization |
| COVID-19 prophylaxis studies | |||||||||
| Chahla 2021142 | Argentina | Open-label | None reported | Health care workers | 234 | 12 mg (in drops) weekly + iota-carrageenan 6 sprays daily × 4 wk | SOC | Prepublication data/manuscript in progress obtained via email | COVID-19 infection (not clear if measured by PCR or symptoms) |
| Elgazzar 202047 | Egypt | Open-label | Self-funded | Health care and family contacts | 200 | 0.4 mg/kg, weekly × 2 weeks | SOC | Research square preprint: emailed/responded with data | Positive PCR test |
| Shouman 2020143 | Egypt | Open-label | Self-funded | Family contacts | 304 | 2 doses (15–24 mg depending on weight) on day 1 and day 3 | SOC | Published in PR journal | Symptoms and/or positive COVID-19 PCR test within 14 days; adverse events |
SOC, standard of care; PR, peer review.
A risk-of-bias summary graph is given in Figure Figure2.2. Eleven studies23,24,44,47,105,106–111 used satisfactory random sequence generation and allocation concealment. Two trials described satisfactory sequence generation, but it was unclear whether allocation was concealed.112,113
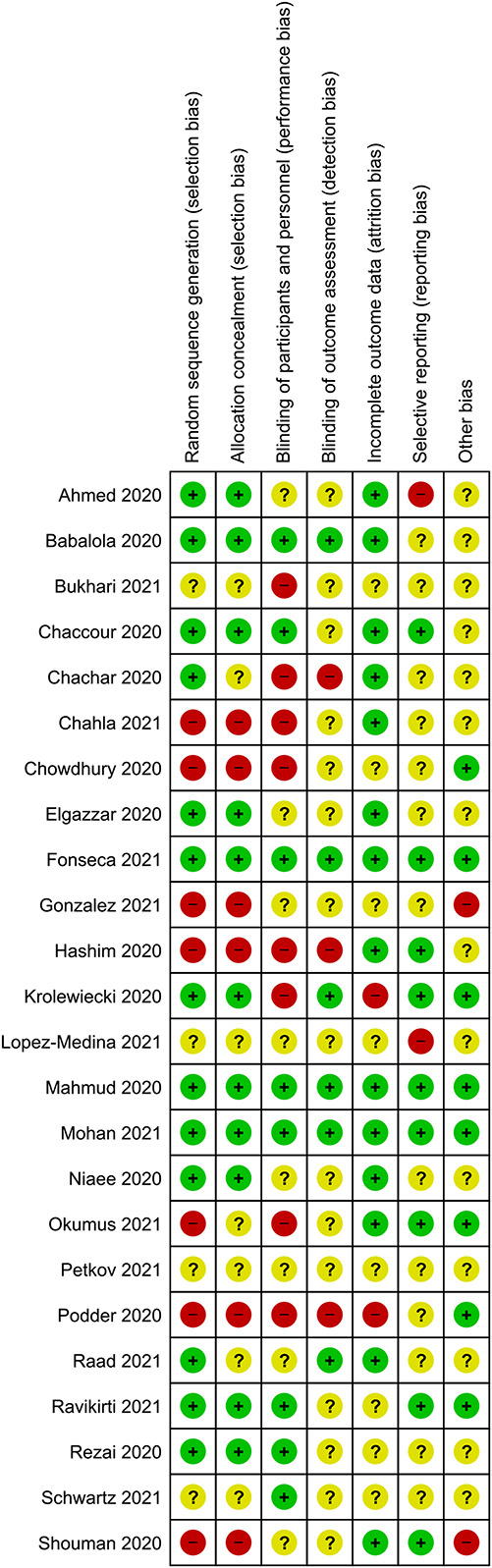
Risk-of-bias summary: review authors’ judgments about each risk of bias item for each included study.
Ten trials reported adequate blinding of the participants/personnel and/or the outcome assessors.23,24,44,105,107,109,110,111,113,114 The others were either unclear or high risk for blinding. We considered blinding to be a less important criterion for evaluation of evidence related to the review’s primary outcomes, namely death and laboratory-confirmed COVID-19 infection, which are objective outcomes.
We did not consider publication on preprint web sites to constitute a risk of bias because all studies were scrutinized and peer reviewed by us during the review process and, where additional information was needed, we contacted the authors for clarification.
Main findings
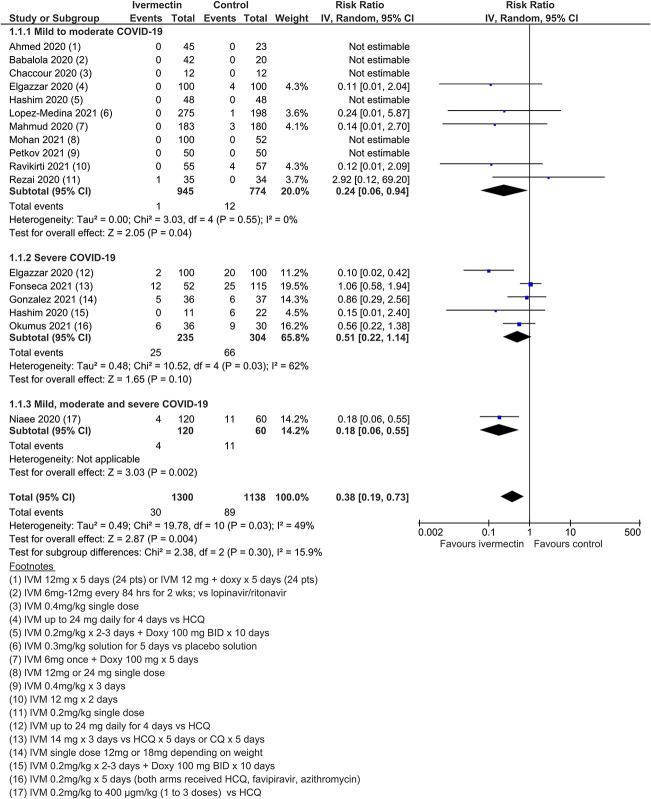
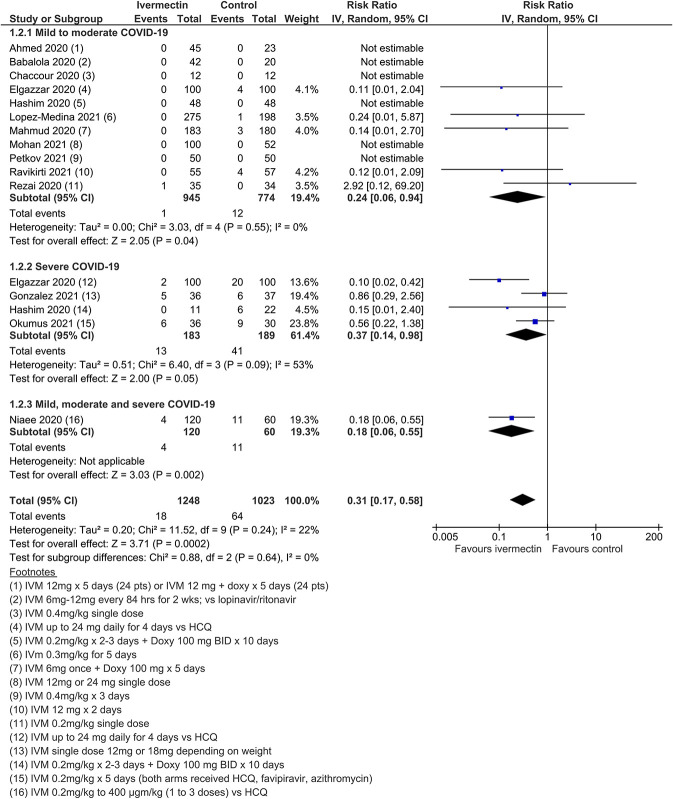
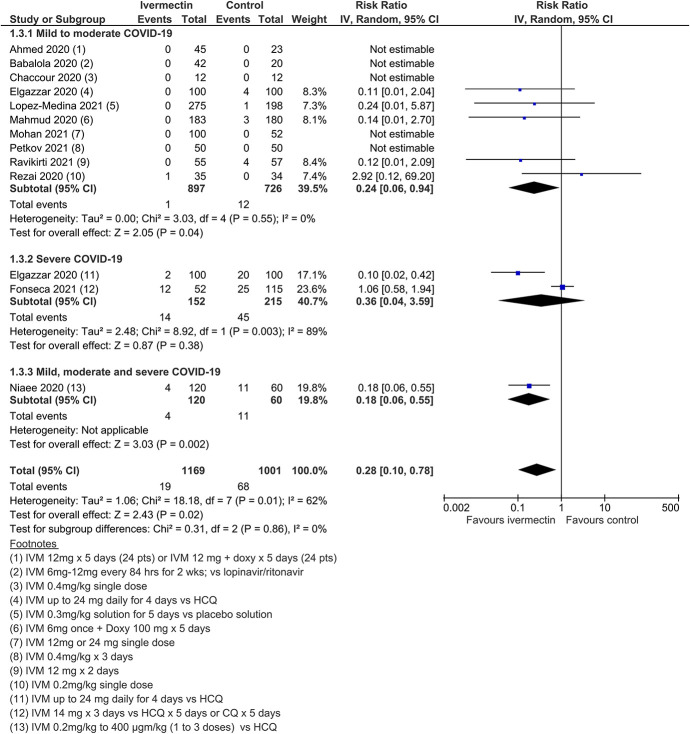
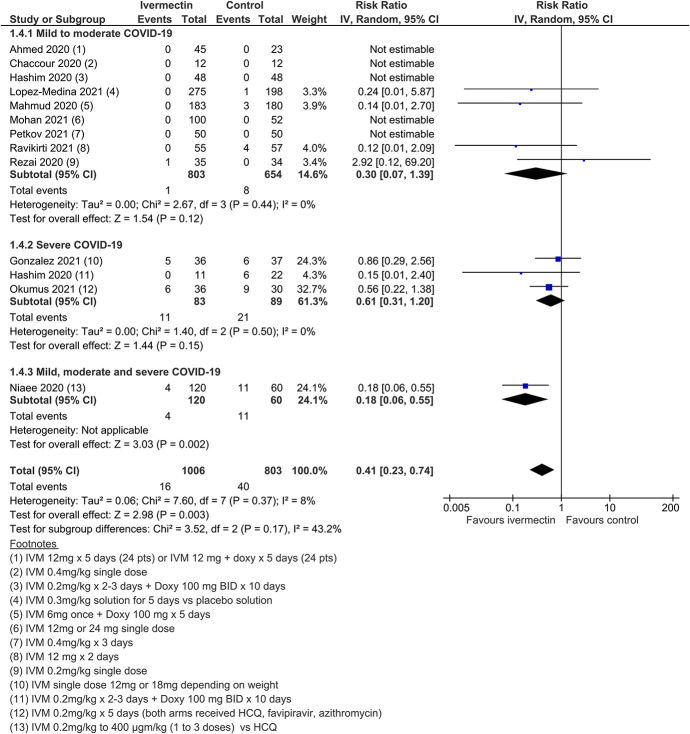
Twenty-four RCTs (including 3 quasi-RCTs) involving 3406 participants were included, with sample sizes ranging from 24 to 476 participants. Twenty-two trials in treatment and 3 trials in prophylaxis met review inclusion, including the trial of Elgazzar et al, which reported both components. For trials of COVID-19 treatment, 16 evaluated ivermectin among participants with mild to moderate COVID-19 only; 6 trials included patients with severe COVID-19. Most compared ivermectin with placebo or no ivermectin; 3 trials included an active comparator (Table (Table1).1). Three RCTs involving 738 participants were included in the prophylaxis trials. Most trials were registered, self-funded, and undertaken by clinicians working in the field. There were no obvious conflicts of interest noted, with the exception of two trials.85,139
Ivermectin treatment versus no ivermectin treatment
Twenty-two trials (2668 participants) contributed data to the comparison ivermectin treatment versus no ivermectin treatment for COVID-19 treatment.
All-cause mortality
Meta-analysis of 15 trials, assessing 2438 participants, found that ivermectin reduced the risk of death by an average of 62% (95% CI 27%–81%) compared with no ivermectin treatment [average RR (aRR) 0.38, 95% CI 0.19 to 0.73; I2 = 49%]; risk of death 2.3% versus 7.8% among hospitalized patients in this analysis, respectively (SoF Table Table22 and Figure Figure3).3). Much of the heterogeneity was explained by the exclusion of one trial44 in a sensitivity analysis (average RR 0.31, 95% CI 0.17–0.58, n = 2196, I2 = 22%), but because this trial was at low risk of bias, it was retained in the main analysis. The source of heterogeneity may be due to the use of active comparators in the trial design. The results were also robust to sensitivity analyses excluding 2 other studies with an active treatment comparator (average RR 0.41, 95% CI 0.23–0.74, n = 1809, I2 = 8%). The results were also not sensitive to the exclusion of studies that were potentially at higher risk of bias (average RR 0.29, 95% CI 0.10–0.80, 12 studies, n = 2095, I2 = 61%), but in subgroup analysis, it was unclear as to whether a single dose would be sufficient. The effect on reducing deaths was consistent across mild to moderate and severe disease subgroups. Subgrouping data according to inpatient and outpatient trials was not informative because few outpatient studies reported this serious outcome. The conclusions of the primary outcome were also robust to a series of alternative post hoc analyses that explored the impact of numerous trials that reported no deaths in either arm. Extreme sensitivity analyses using a treatment arm continuity correction of between 0.01 and 0.5 did not change the certainty of the evidence judgments (Table (Table33).
Table 2.
Summary of findings table of ivermectin versus no ivermectin for COVID-19 treatment in any setting.
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |||
| Assumed risk | Corresponding risk | ||||||
| No ivermectin | Ivermectin | ||||||
| Death from any cause | 78 per 1000 (all disease severity) | 48 fewer deaths per 1000 (21–63) | RR = 0.38 (0.19–0.73) | 2438 (15) | Moderate† | ||
| Recovery time to negative PCR test, in days | Absolute risks were not computed due to certainty of evidence being low and, in some cases, number of events being sparse | MD = −3.20 (−5.99 to −0.40) | 375 (6) | Very low†,‡,§ | |||
| Time to clinical recovery, in days (outpatients) | MD = −1.06 (−1.63 to −0.49) | 176 (2) | Very low†,‡,§ | ||||
| Time to clinical recovery, in days (mild to moderate COVID-19 inpatients) | MD = −7.32 (−9.25 to −5.39) | 96 (1) | Very low†,¶ | ||||
| Time to clinical recovery, in days (severe COVID-19 inpatients) | MD = −3.98 (−10.06 to 2.10) | 33 (1) | Very low†,¶ | ||||
| Admission to ICU | RR=1.22 (0.75–2.00) | 379 (2) | Very low¶,║¦ | ||||
| Need for mechanical ventilation | RR=0.66 (0.14–3.00) | 431 (3) | Low§,║ | ||||
| Length of hospital stay, in days | MD= 0.13 (−2.04 to 2.30) | 68 (1) | Very low†,¶ | ||||
| Admission to hospital | RR 0.16 (0.02–1.32) | 194 (2) | Very low†,¶ | ||||
| Duration of mechanical ventilation | Not reported | ||||||
| Improvement (mild to moderate COVID-19)* | 635 improved per 1000 | 159 more per 1000 (from 51 more to 286 more) | RR 1.25 (1.08–1.45) | 681 (5) | Low†,‡ | ||
| Deterioration (any disease severity) | 143 per 1000 | 93 fewer per 1000 (from 50 fewer to 116 fewer) | RR 0.35 (0.19–0.65) | 1587 (7) | Low†,‡ | ||
| Serious adverse events | 7/867 (0.8%) had an SAE in ivermectin group and 2/666 (0.3%) in control | RR=1.65 (0.44–6.09) | 1533 (11) | Low†,‡ | |||
Table 3.
Sensitivity analyses for death from any cause considering methods for dealing with zero events in trials.
| Method | Measure | Model | Effect size (95% CI) | Details |
| Peto | OR | FE | 0.35 (0.24 to 0.53) | Handles single-zero trials |
| M-H | OR | FE | 0.37 (0.24 to 0.56) | Handles single-zero trials |
| M-H | OR | RE | 0.33 (0.16 to 0.68) | Handles single-zero trials |
| M-H | RR | FE | 0.42 (0.29 to 0.60) | Handles single-zero trials |
| M-H | RR | RE | 0.37 (0.19 to 0.74) | Handles single-zero trials |
| M-H | RD | FE | −0.04 (−0.06 to −0.02) | Handles double-zero trials |
| M-H | RD | RE | −0.03 (−0.06 to −0.00) | Handles double-zero trials |
| IV | RD | FE | −0.01 (−0.02 to −0.00) | Handles double-zero trials |
| IV | RD | RE | −0.02 (−0.04 to −0.00) | Handles double-zero trials |
| Treatment arm continuity correction methods using IV | Accounting for double zeros | Accounting for all zeros | ||
| 0.01 | RR | FE | 0.54 (0.36 to 0.79) | 0.58 (0.39–0.88) |
| 0.01 | RR | RE | 0.43 (0.25 to 0.72) | 0.58 (0.39–0.88) |
| 0.1 | RR | FE | 0.54 (0.37 to 0.79) | 0.56 (0.38–0.84) |
| 0.1 | RR | RE | 0.43 (0.26 to 0.73) | 0.46 (0.26–0.80) |
| 0.25 | RR | FE | 0.54 (0.37 to 0.79) | 0.55 (0.37–0.81) |
| 0.25 | RR | RE | 0.44 (0.26 to 0.73) | 0.45 (0.26–0.76) |
| 0.5 | RR | FE | 0.54 (0.37 to 0.79) | 0.55 (0.35–0.78) |
| 0.5 | RR | RE | 0.45 (0.27 to 0.74) | 0.47 (0.29–0.75) |
FE, fixed effects; IV, inverse variance; M-H, Mantel-Haenszel; RD, risk difference; RE, random effects; TACC, treatment arm continuity correction.
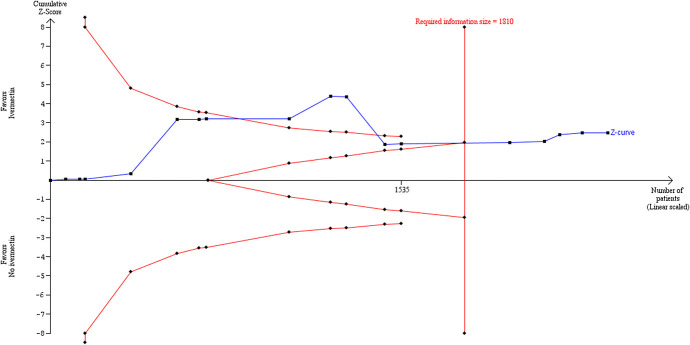
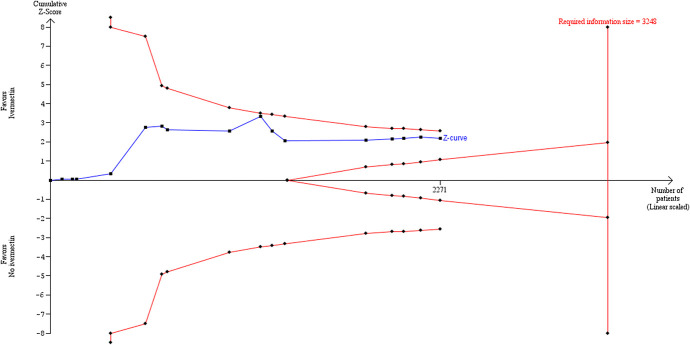
Trial sequential analysis
TSA, using the DL random-effects method, showed that there may have been sufficient evidence accrued before the end of 2020 to show significant benefit of ivermectin over control for all-cause mortality. The cumulative z-curve in Figure Figure88 crossed the trial sequential monitoring boundaries after reaching the required IS, implying that there is firm evidence for a beneficial effect of ivermectin use over no ivermectin use in mainly hospitalized participants with mild to moderate COVID-19 infection.
Trial sequential analysis using DL random-effects method with parameter estimates of α = 0.05, β = 0.1, control rate = 7.8%, RRR = 62%, and diversity = 49.5%.
The TSA was used to calculate the IS required to demonstrate or reject a 62% RRR of death in the ivermectin group, as observed in the primary meta-analysis. This estimate is similar to effect estimates reported in other reviews.10 We assumed a 7.8% event proportion in the control group, which was the average control group event rate from the primary meta-analysis. We used a model variance-based estimate of 49.1% (diversity estimate) to correct for heterogeneity. The required IS was 1810 participants (Figure (Figure8),8), which was exceeded by the total number of observed participants in the meta-analysis (n = 2438). In the TSA plots, the red dashed lines in Figure Figure88 represent the trial sequential monitoring boundaries using the O’Brien–Fleming alpha-spending function. The solid blue line is the cumulative z-curve and represents the observed trials in the cumulative meta-analysis. The adjusted significance boundaries for the cumulative z-curve were constructed under the assumption that significance testing may have been performed each time a new trial was added to the meta-analysis. In Figure Figure8,8, the z-curve crosses the boundary after reaching the required IS, thereby supporting the previous conclusion in RevMan 5.4.131 using the DL method that ivermectin is superior to control in reducing the risk of death.
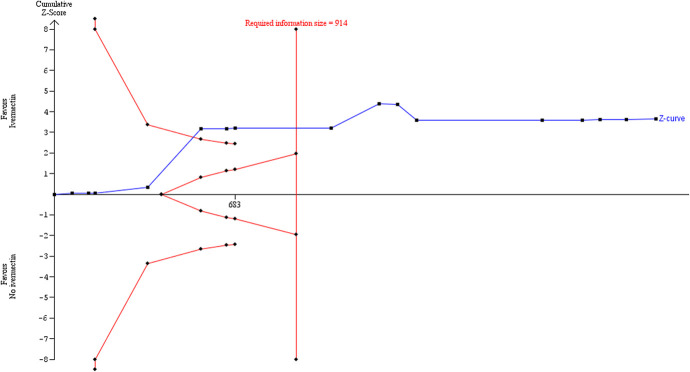
Sensitivity analyses
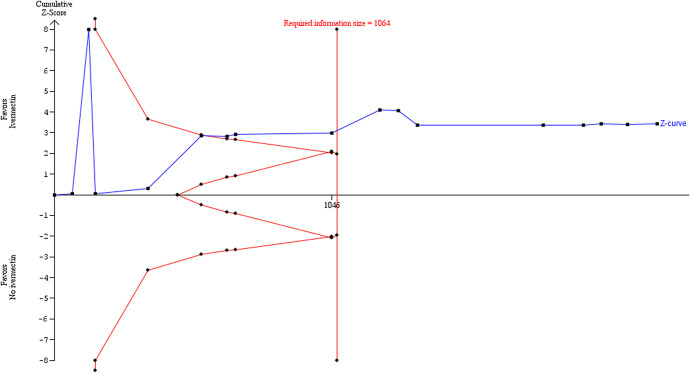
Sensitivity analysis excluding the trial of Fonseca44 significantly reduced heterogeneity in the meta-analysis and thus the diversity estimate in the TSA using the DL model. This strengthened the suggestion in the primary core analysis that the required IS had been reached (Figure (Figure9).9). Because the DL estimator could potentially underestimate the between-trials variance,43 we performed further sensitivity analyses using 2 alternative random-effects model approaches. The results of the primary TSA analysis were robust to sensitivity analysis using the BT method with the same parameters, excluding the Fonseca44 trial, which was a cause of substantial heterogeneity (Figure (Figure10).10). The TSA comprehensively confirms the result of the conventional meta-analysis. The required IS was 1064.
Sensitivity analysis excluding an outlier study responsible for the heterogeneity, showing trial sequential analysis using DL random-effects method with parameter estimates of α = 0.05, β = 0.1, control rate = 7.8%, = 62%, and diversity = 0%.
Sensitivity analysis excluding an outlier study responsible for the heterogeneity, showing trial sequential analysis using Biggerstaff–Tweedie random-effects method with parameter estimates of α = 0.05, β = 0.1, control rate = 7.8%, RRR = 62%, and diversity = 14.2%.
The required IS was not reached in the TSA using the SJ method, largely because diversity from the model was high (Figure (Figure11).11). The SJ estimator may overestimate the between-trials variance in meta-analyses with mild heterogeneity, thus producing artificially wide confidence intervals.43 When the diversity estimate was reduced to the same as in the DL model, the required IS was reached in the SJ model (data not shown). There was no evidence of futility using the SJ method in any scenario.
Sensitivity analysis excluding an outlier study responsible for the heterogeneity, showing trial sequential analysis using Sidik–Jonkman random-effects method with parameter estimates of α = 0.05, β = 0.1, control rate = 7.8%, RRR = 62%, and diversity = 71.9%.
Certainty of the evidence for all-cause mortality
Overall, death from any cause, taking into account all composite analyses, was judged to provide moderate-certainty evidence (SoF Table Table22 and Figures Figures44–11). A funnel plot corresponding to the primary outcome of death from any cause did not seem to suggest any evidence of publication bias (Figure (Figure7).7). Furthermore, the ease with which trial reports can be uploaded as preprints should reduce this risk.
Death due to any cause, excluding an outlier study responsible for the heterogeneity.
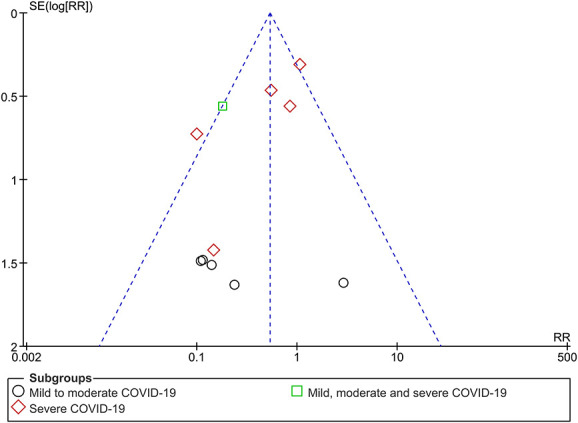
Funnel plot of ivermectin versus control for COVID-19 treatment for all-cause death (subgrouped by severity).
Secondary outcomes
Secondary outcomes provided low to very low certainty evidence (SoF Table Table2).2). Low-certainty findings suggested that there may be no benefit with ivermectin for “need for mechanical ventilation,” whereas effect estimates for “improvement” and “deterioration” favored ivermectin but were graded as low certainty due to study design limitations and inconsistency (Figures (Figures1212–14). All other secondary outcome findings were assessed as very low certainty.
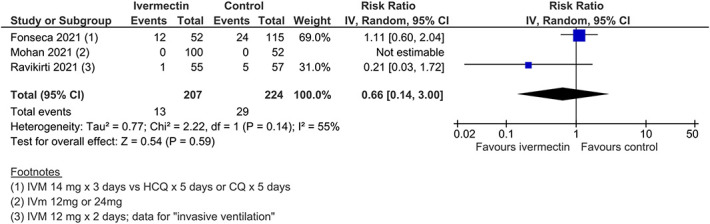
Need for mechanical ventilation.
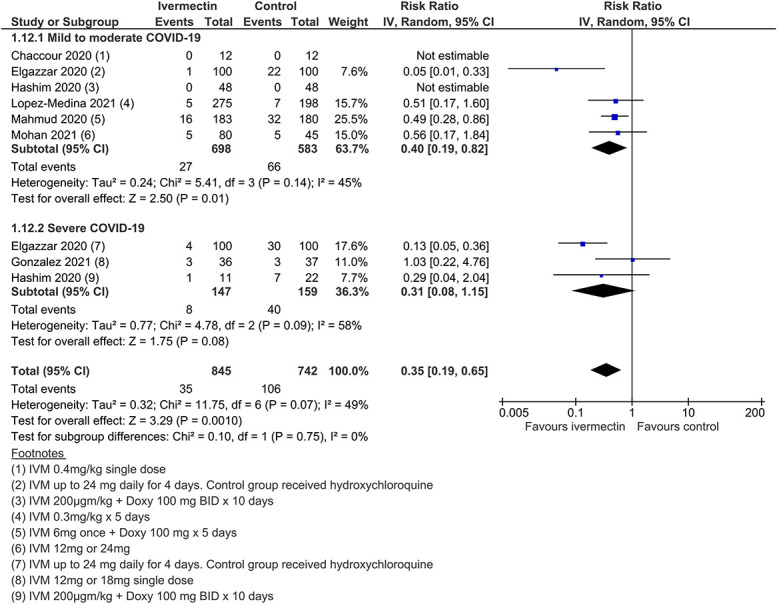
Deterioration.
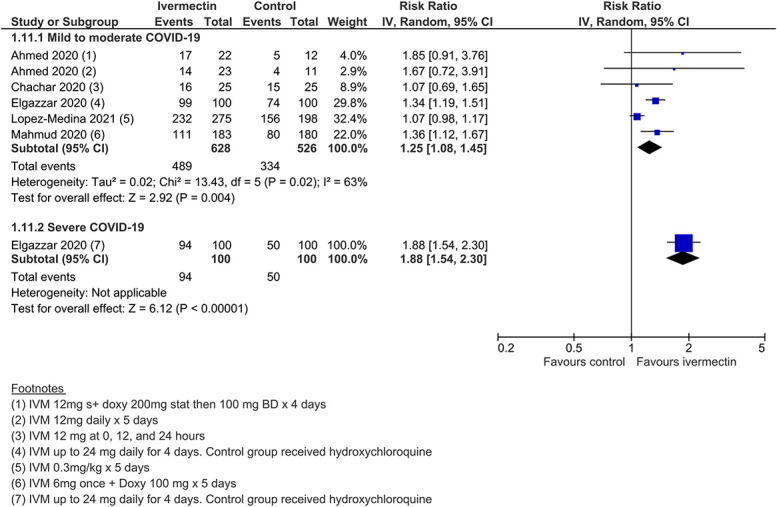
Improvement.
Meta-analysis of 11 trials, assessing 1533 participants, found that there was no significant difference between ivermectin and control in the risk of severe adverse events (aRR 1.65, 95% CI 0.44–6.09; I2 = 0%; low certainty evidence, downgraded for imprecision and study design limitations). Seven severe adverse events were reported in the ivermectin group and 2 in controls. The SAEs were as follows: 2 patients in the Mahmud trial107 had esophagitis (this is a known side effect of doxycycline, which was coadministered with ivermectin in this trial); one patient in the study by Krolewiecki et al106 had hyponatremia (this trial used high-dose ivermectin for 5 days); and 2 patients in a study from Turkey115 had serious “delirium-like behavior, agitation, aggressive attitude, and altered state of consciousness,” which the authors attributed to metabolic insufficiencies in MDR-1/ABCB1 or CYP3A4 genes, screening for which was a study feature. In the Lopez-Medina et al85 trial, there were 2 SAEs in each arm (SoF Table Table22).
Ivermectin prophylaxis versus no ivermectin prophylaxis
Three studies involving 738 participants evaluated ivermectin for COVID-19 prophylaxis among health care workers and COVID-19 contacts. Meta-analysis of these 3 trials, assessing 738 participants, found that ivermectin prophylaxis among health care workers and COVID-19 contacts probably reduces the risk of COVID-19 infection by an average of 86% (79%–91%) (3 trials, 738 participants; aRR 0.14, 95% CI 0.09–0.21; 5.0% vs. 29.6% contracted COVID-19, respectively; low-certainty evidence; downgraded due to study design limitations and few included trials) (Figure (Figure15).15). In 2 trials involving 538 participants, no severe adverse events were recorded (SoF Table Table44).
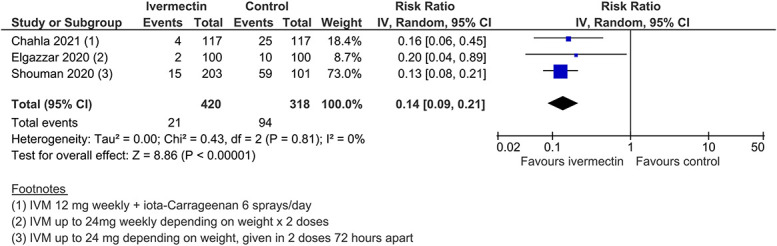
COVID-19 infection (prophylaxis studies).
Table 4.
Summary of findings table of ivermectin versus no ivermectin for COVID-19 prophylaxis in healthy population (people without COVID-19 infection).
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | ||
| Assumed risk | Corresponding risk | |||||
| No ivermectin | Ivermectin | |||||
| COVID-19 infection | 296 per 1000 | 245 fewer infections per 1000 (234–269) | RR = 0.14 (0.09–0.21) | 738 (3) | Low† | |
| Admission to hospital | Not reported | |||||
| Death from any cause | Not reported | |||||
| Serious adverse events | No events occurred in 538 participants (2 studies), therefore the effect could not be estimated. | |||||
GRADE working group grades of evidence; High quality: Further research is very unlikely to change our confidence in the estimate of effect; Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality: We are very uncertain about the estimate.
NNT, number needed to treat.
DISCUSSION
The findings indicate with moderate certainty that ivermectin treatment in COVID-19 provides a significant survival benefit. Our certainty of evidence judgment was consolidated by the results of trial sequential analyses, which show that the required IS has probably already been met. Low-certainty evidence on improvement and deterioration also support a likely clinical benefit of ivermectin. Low-certainty evidence suggests a significant effect in prophylaxis. Overall, the evidence also suggests that early use of ivermectin may reduce morbidity and mortality from COVID-19. This is based on (1) reductions in COVID-19 infections when ivermectin was used as prophylaxis, (2) the more favorable effect estimates for mild to moderate disease compared with severe disease for death due to any cause, and (3) on the evidence demonstrating reductions in deterioration.
The evidence on severe adverse events in this review was graded as low certainty, partly because there were too few events to reach statistical significance. Evidence from a recent systematic review of ivermectin use among people with parasitic infections suggests that ivermectin administered at the usual doses (0.2 or 0.4 mg/kg) is safe and could be safe at higher doses.7,116 A recent World Health Organization document on ivermectin use for scabies found that adverse events with ivermectin were primarily minor and transient.22
We restricted the included studies to the highest level of evidence, that is, RCTs, as a policy. This was despite there being numerous observational but nonrandomized trials of ivermectin, which one could argue could also be considered in an emergency. We included preprint and unpublished data from completed but not yet published trials due to the urgency related to evidence synthesis in the context of a global pandemic.117 Although there is the potential for selective reporting of outcomes and publication bias, we have factored in these considerations in interpreting results and forming conclusions. We adhered to PRISMA guidelines and the WHO statement on developing global norms for sharing data and results during public health emergencies.117
There are a number of limitations with this review. Several of the studies contributing data did not provide full descriptions of methods, so assessing risk of bias was challenging. Where descriptions of study methods were sparse or unclear, we attempted to contact authors to clarify methods, but lack of information led us to downgrade findings in several instances. Overall interpretation of findings was hampered due to variability in the participants recruited, treatment regimen, and the care offered to those in control groups. We have tried to take this variation into account through subgroup and sensitivity analyses. Nevertheless, dosing and treatment regimens and the use of ivermectin with other components of “standard care” require further research. We did not include laboratory outcome measures, such as viral clearance. The latter and other biochemical outcomes have been reported in several studies and reviews and tend to favor ivermectin.10,47,105,108 Several trials reported continuous data, such as length of hospital stay, as medians and interquartile ranges; therefore, we were unable to include these data in meta-analysis. Because we did not undertake in our protocol to perform narrative evidence synthesis, and because these data tended to favor ivermectin, the certainty of the effects of ivermectin on these continuous outcomes may be underestimated.
At least 5 other reviews of ivermectin use for COVID-19 have been published, including one coauthored with Nobel Laureate Professor Satoshi Ōmura, discoverer of ivermectin,9,10,118,119,120 but only 3 have been peer-reviewed9,118,120 and only 2 attempt full systematic review.10,119 We applied AMSTAR 2,121 a critical appraisal tool for systematic reviews of health care interventions, to the 2 nonpeered systematic reviews10,119 and both were judged to be of low quality (Table (Table5).5). However, there was also a suggestion that ivermectin reduced the risk of death in treatment of COVID-19 in these reviews.
Table 5.
Methodological quality of other systematic reviews (AMSTAR 2).
| Systematic review | Components of PICO described | A priori study design | Explain selection of study designs | Comprehensive literature search | Duplicate study selection | Duplicate data extraction | List of excluded studies justified | Characteristics of included studies provided |
| Hill et al, 202110 | + | − | + | + | ? | ? | −* | ?† |
| Castañeda-Sabogal et al 2021119 | +║ | ? | − | ?# | + | + | −* | + |
| Systematic review | Risk of bias adequately assessed and documented | Sources of funding reported | Appropriate methods to combine findings | Appropriate risk-of-bias sensitivity analyses conducted | Risk-of-bias assessment used in conclusions | Satisfactory explanation of observed heterogeneity | Likelihood of publication bias assessed | Conflict of interest stated |
| Hill et al, 202110 | −‡ | − | −§ | −* | −¶ | −* | NA | − |
| Castañeda-Sabogal et al 2021119 | −** | − | −†† | −‡‡ | −* | + | NA | + |
Assessed using AMSTAR 2121; +, adequately assessed; −, inadequately assessed; ?, unclear assessment; NA, not applicable (less than 10 included studies in meta-analysis).
The recently updated WHO therapeutics guidelines12 included 7 trials and 1419 people in the analysis of mortality. Reporting a risk reduction of 81% (odds ratio 0.19, 95% CI 0.09–0.36), the effect estimate favoring ivermectin was downgraded by 2 levels for imprecision, although the justification for this is unclear as the reported CI is precise (64%–91%).
In addition to the evidence from systematic reviews, the findings of several controlled observational studies are consistent with existing evidence and suggest improved outcomes with ivermectin treatment.55,57,59 Similarly, with respect to ivermectin prophylaxis of frontline workers and those at risk, controlled observational studies from Bangladesh and Argentina (the latter which involved 1195 health care workers) have shown apparent reductions in COVID-19 transmission with ivermectin prophylaxis, including in some reports total protection (zero infections) where infection rates in the control group exceeded 50%.122,123 A very large trial of ivermectin prophylaxis in health care workers in India124 covered 3532 participants and reported risk ratios not significantly different from this meta-analysis (prophylaxis outcome).
Clarifying ivermectin safety in pregnancy is a key question in patient acceptability for pregnant women contracting COVID-19. A recent meta-analysis5 found little evidence of increased risk of abnormal pregnancies but similarly weak evidence of absence of risk. For (pre-exposure) prophylaxis in pregnancy, where vaccines may be contraindicated, the alternative of hydroxychloroquine has been advocated.125,126 In addition to safety and relative efficacy, different risk–benefit judgments may be presented for prophylaxis (pre- and post-exposure), and for treatment, with pregnancy a high-risk status for COVID-19.
RCTs in this review did not specifically examine use of ivermectin in the elderly, although this is a known high-risk group for severe COVID-19. In the setting of care homes, it is also notorious for rapid contagion. A standard indication for ivermectin in the elderly is scabies. We identified 2 recent reports suggesting that ivermectin may be efficacious as prevention and treatment of COVID-19 in this age group.50,127 A letter on positive experience in 7 elder care facilities in Virginia covering 309 patients was sent to NIH127 and has recently been submitted for publication.
There is also evidence emerging from countries where ivermectin has been implemented. For example, Peru had a very high death toll from COVID-19 early on in the pandemic.128 Based on observational evidence, the Peruvian government approved ivermectin for use against COVID-19 in May 2020.128 After implementation, death rates in 8 states were reduced between 64% and 91% over a two-month period.128 Another analysis of Peruvian data from 24 states with early ivermectin deployment has reported a drop in excess deaths of 59% at 30+ days and of 75% at 45+ days.129 However, factors such as change in behavior, social distancing, and face-mask use could have played a role in this reduction.
Other considerations related to the use of ivermectin treatment in the COVID-19 pandemic include people’s values and preferences, equity implications, acceptability, and feasibility.130 None of the identified reviews specifically discussed these criteria in relation to ivermectin. However, in health care decision making, evidence on effectiveness is seldom taken in isolation without considering these factors. Ultimately, if ivermectin is to be more widespread in its implementation, then some considerations are needed related to these decision-making criteria specified in the GRADE-DECIDE framework.130
There are numerous emerging ongoing clinical trials assessing ivermectin for COVID-19. The trade-off with policy and potential implementation based on evidence synthesis reviews and/or RCTs will vary considerably from country to country. Certain South American countries, Indian states, and, more recently, Slovakia and other countries in Europe have implemented its use for COVID-19.129,131,132,133,134 A recent survey of global trends118 documents usage worldwide. Despite ivermectin being a low-cost medication in many countries globally, the apparent shortage of economic evaluations indicates that economic evidence on ivermectin for treatment and prophylaxis of SARS-CoV-2 is currently lacking. This may impact more on LMICs that are potentially waiting for guidance from organizations like the WHO.
Given the evidence of efficacy, safety, low cost, and current death rates, ivermectin is likely to have an impact on health and economic outcomes of the pandemic across many countries. Ivermectin is not a new and experimental drug with an unknown safety profile. It is a WHO “Essential Medicine” already used in several different indications, in colossal cumulative volumes. Corticosteroids have become an accepted standard of care in COVID-19, based on a single RCT of dexamethasone.1 If a single RCT is sufficient for the adoption of dexamethasone, then a fortiori the evidence of 2 dozen RCTs supports the adoption of ivermectin.
Ivermectin is likely to be an equitable, acceptable, and feasible global intervention against COVID-19. Health professionals should strongly consider its use, in both treatment and prophylaxis.
ACKNOWLEDGMENTS
This work was inspired by the prior literature review of Dr Pierre Kory.
The authors thank Information Specialist, Jo Platt, of the Cochrane Gynaecological, Neuro-oncology, and Orphan Cancer (CGNOC) group for designing the search strategy and running the search, as well as Anna Noel Storr for reviewing the strategy. The authors also thank Isabella Rushforth for her voluntary assistance with the preparation of the reference lists.
The authors thank Gill Gyte for detailed comments, feedback, and involvement in this review, and Michael Grayling and David Tovey for useful peer review comments before submission. The authors also thank the external peer reviewers for their helpful comments and Peter Manu for the opportunity to publish our findings.
Footnotes
The preprint of this review received no funding. This updated version was funded by the crowdfunding initiative https://www.gofundme.com/f/help-us-get-lifesaving-drug-approved-for-covid19
The authors have no conflicts of interest to declare.
T. A. Lawrie and A. Bryant cowrote the review; they also sifted the search and classified studies for inclusion and entered and checked the data in RevMan and performed analyses. Data extraction was divided among T. A. Lawrie, A. Bryant, and T. Dowswell. T. Dowswell and A. Bryant graded the evidence. E. J. Fordham prepared the text on ivermectin mechanisms, use in pregnancy, and among the elderly. S. R. Hill prepared the brief economic commentary. Clinicians S. Mitchell and T. C. Tham contributed to the interpretation of the evidence in the discussion and conclusions. All authors reviewed and approved the final version of the manuscript.
This article discusses off-label use of the FDA-approved medication ivermectin against COVID-19.