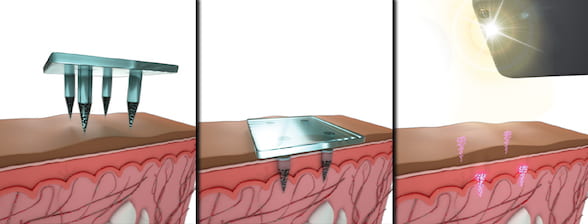
Quantum-dot tattoos hold vaccination record
Mike Williams – Dec. 18, 2019 POSTED IN: RICE NEWS > Current News > 2019 Quantum-dot tattoos hold vaccination record Rice bioengineer reveals dissolving microneedles that also embed fluorescent medical info Keeping track of a child’s shots could be so much easier with technology invented by a new Rice University professor and his colleagues. Kevin McHugh, an assistant professor of bioengineering at … Read more