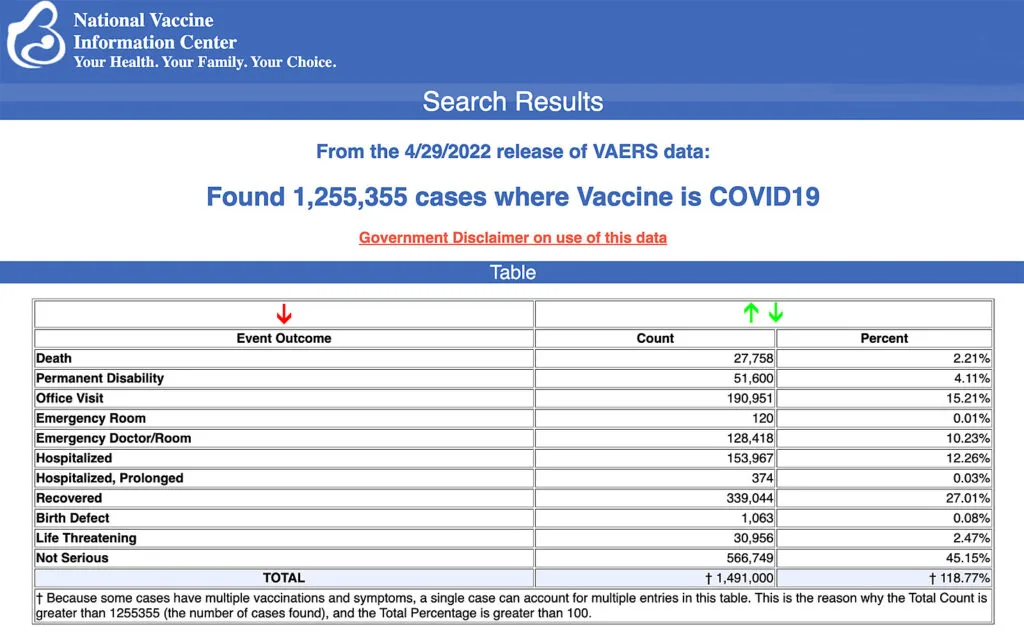
VAERS data released Friday by the Centers for Disease Control and Prevention included a total of 1,255,355 reports of adverse events from all age groups following COVID-19 vaccines, including 27,758 deaths and 226,703 serious injuries between Dec. 14, 2020, and April 29, 2022.

The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,255,355 reports of adverse events following COVID-19 vaccines were submitted between Dec. 14, 2020, and April 29, 2022, to the Vaccine Adverse Event Reporting System (VAERS). VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
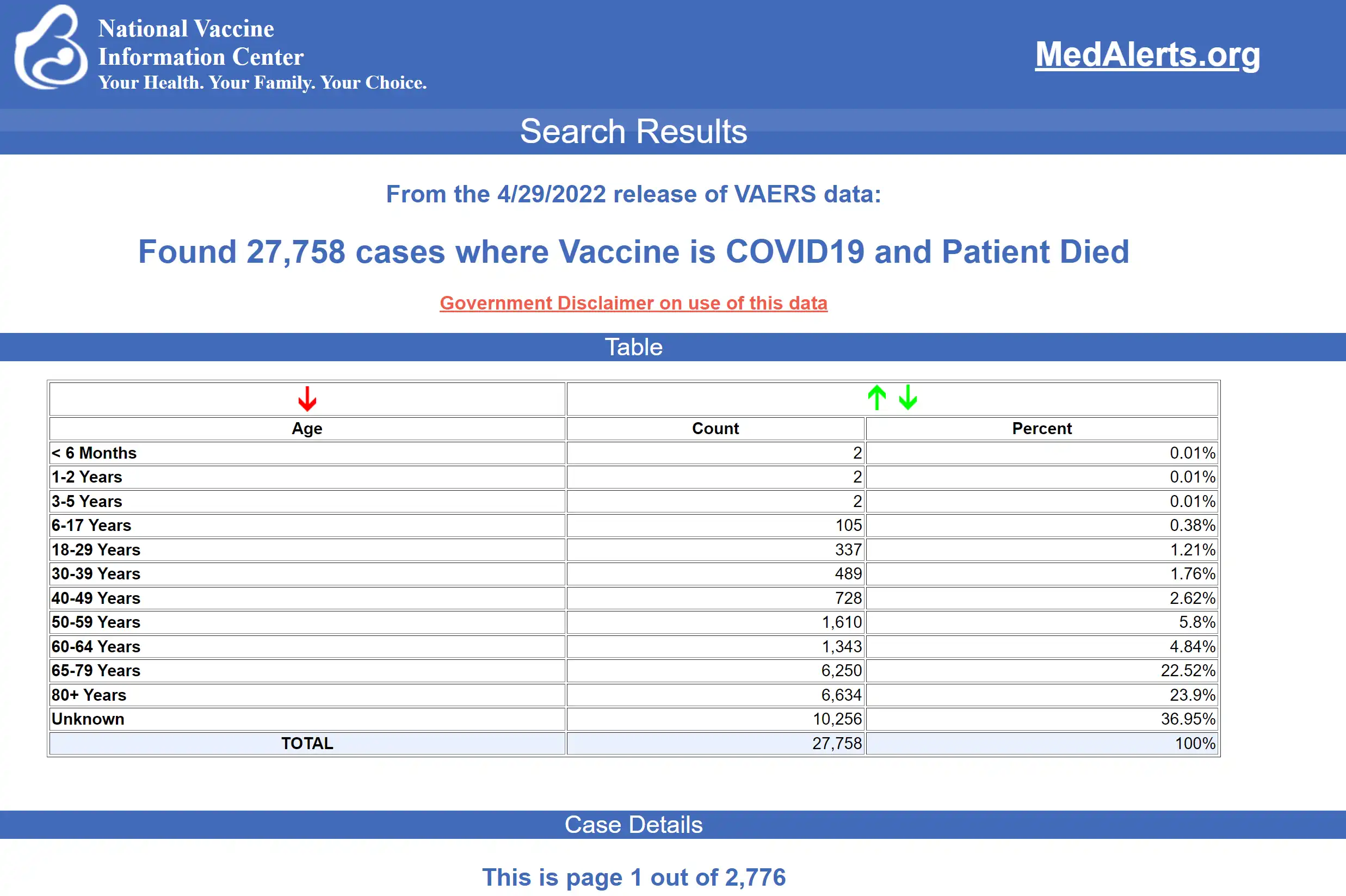
The data included a total of 27,758 reports of deaths — an increase of 226 over the previous week — and 226,703 serious injuries, including deaths, during the same time period — up 1,937 compared with the previous week. There were 8,224 additional total adverse events reported to VAERS over the previous week.
Excluding “foreign reports” to VAERS, 813,021 adverse events, including 12,779 deaths and 81,271 serious injuries, were reported in the U.S. between Dec. 14, 2020, and April 29, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 12,779 U.S. deaths reported as of April 29, 16% occurred within 24 hours of vaccination, 20% occurred within 48 hours of vaccination and 59% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 575 million COVID-19 vaccine doses had been administered as of April 29, including 339 million doses of Pfizer, 217 million doses of Moderna and 19 million doses of Johnson & Johnson (J&J).
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S. VAERS data from Dec. 14, 2020, to April 29, 2022, for 5- to 11-year-olds show:
- 10,444 adverse events, including 261 rated as serious and 5 reported deaths.
- 19 reports of myocarditis and pericarditis (heart inflammation). The CDC uses a narrowed case definition of “myocarditis,” which excludes cases of cardiac arrest, ischemic strokes and deaths due to heart problems that occur before one has the chance to go to the emergency department. The Defender has noticed over previous weeks that several reports of myocarditis and pericarditis have been removed by the CDC from the VAERS system in this age group. No explanation was provided.
- 43 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to April 29, 2022, for 12- to 17-year-olds show:
- 31,504 adverse events, including 1,808 rated as serious and 44 reported deaths.
The most recent reported death involves a 14-year-old girl from Tennessee (VAERS I.D. 2238618) who died after receiving her second dose of Pfizer’s COVID-19 vaccine. According to the VAERS report, the girl had a previous history of cancer but was hospitalized 29 days after receiving her second dose of Pfizer with severe COVID-19 and COVID pneumonia. She became “critically ill,” developed respiratory failure and bradycardia and later died. - 65 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 96% of cases attributed to Pfizer’s vaccine.
- 650 reports of myocarditis and pericarditis — two fewer than last week — with 638 cases attributed to Pfizer’s vaccine.
- 166 reports of blood clotting disorders — 1 fewer than last week — with all cases attributed to Pfizer.
U.S. VAERS data from Dec. 14, 2020, to April 29, 2022, for all age groups combined, show:
- 20% of deaths were related to cardiac disorders.
- 54% of those who died were male, 41% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 73.
- As of April 29, 5,480 pregnant women reported adverse events related to COVID-19 vaccines, including 1,711 reports of miscarriage or premature birth.
- Of the 3,626 cases of Bell’s Palsy reported — seven fewer cases than what was reported two weeks ago — 51% were attributed to Pfizer vaccinations, 40% to Moderna and 8% to J&J.
- 872 reports of Guillain-Barré syndrome, with 42% of cases attributed to Pfizer, 30% to Moderna and 29% to J&J.
- 2,331 reports of anaphylaxis — 24 fewer reports than was what recorded two weeks ago — where the reaction was life-threatening, required treatment or resulted in death.
- 1,692 reports of myocardial infarction.
- 13,873 reports of blood-clotting disorders in the U.S. Of those, 6,227 reports were attributed to Pfizer, 4,943 reports to Moderna and 2,662 reports to J&J.
- 4,164 cases of myocarditis and pericarditis with 2,552 cases attributed to Pfizer’s, 1,420 cases to Moderna’s and 180 cases to J&J’s COVID-19 vaccine.
FDA limits use of J&J shot over rare blood clotting disorder
The FDA on Thursday put strict limits on the use of the Johnson & Johnson (J&J) COVID-19 vaccine, citing the risk of a blood-clotting condition the agency described as “rare and potentially life-threatening.”
In a statement Thursday, the FDA said the risk of vaccine recipients developing thrombosis with thrombocytopenia syndrome (TTS) after receiving the vaccine “warrants limiting the authorized use of the vaccine.”
The agency described TTS as “a syndrome of rare and potentially life-threatening blood clots in combination with low levels of blood platelets with onset of symptoms approximately one to two weeks following administration of the Janssen [J&J] COVID-19 vaccine.”
Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said limiting the authorized use of the Janssen vaccine “demonstrates the robustness of our safety surveillance systems and our commitment to ensuring that science and data guide our decisions.”
However, Brian Hooker, Ph.D., P.E., Children’s Health Defense chief scientific officer and professor of biology at Simpson University, had a different take on the news.
“It seems like the FDA pays lip service to the fact that the spike protein can cause clotting, and to the widespread reports of clotting, by punishing Janssen, who has become the ‘whipping boy’ of the COVID-19 vaccine manufacturers through the pandemic,” Hooker said.
The decision comes about five months after the CDC recommended mRNA vaccines Moderna and Pfizer over J&J for booster doses.
In April 2021, federal health agencies paused distribution of J&J’s vaccine to investigate reported cases of blood clotting disorders occurring in those who received the shot. But regulators lifted the pause 10 days later and added a warning to instructions for its use.
Recipients of Moderna vaccine may be more likely to suffer repeated infections
A new study suggests recipients of Moderna’s COVID-19 vaccine may be more likely to suffer repeated infections, perhaps indefinitely.
The preprint study found participants in Moderna’s adult trial who received the vaccine and later were exposed to the virus, did not generate antibodies to a key component of the virus as often as those in the placebo group.
The authors’ findings, corroborated by U.K. data that demonstrate the rates of infection are significantly higher in the vaccinated, suggest Moderna knew of this safety signal in 2020 when the vaccine maker was conducting its trials.
The study implies that reduced ability of a vaccinated individual to produce antibodies to other portions of the virus may lead to a greater risk of future infections in the vaccinated compared to the unvaccinated.
Evidence suggests that even after a vaccinated person has a breakthrough infection, that individual still does not acquire the same level of protection against subsequent exposures that an unvaccinated person acquires.
Study of 23 million people shows increased risk of myocarditis after COVID vaccines
A study published in JAMA Cardiology on April 20 involving 23 million people shows a COVID-19 vaccine side effect once labeled as “misinformation” is real.
According to the study, “both first and second doses of mRNA vaccines were associated with increased risk of myocarditis and pericarditis. For individuals receiving 2 doses of the same vaccine, risk of myocarditis was highest among young males (aged 16-24 years) after the second dose.”
Myocarditis is inflammation of the heart muscle that can lead to cardiac arrhythmia and death. Pericarditis is inflammation of the tissue surrounding the heart that can cause sharp chest pain and other symptoms.
Specifically, among young men receiving two doses of the same vaccine, between four and seven excess myocarditis and pericarditis events occurred in 28 days per 100,000 vaccinees after the second dose of the Pfizer vaccine, and between nine and 28 excess myocarditis and pericarditis events occurred per 100,000 vaccinees after the second dose of the Moderna vaccine.
The study concluded, “The risk of myocarditis in this large cohort study was highest in young men after the second SARS-CoV-2 vaccine dose” and recommended, “this risk should be balanced against the benefits of protecting against severe COVID-19 disease.”
CHD California secures two big wins against public school vaccine mandates
Children’s Health Defense, California Chapter (CHD-CA) and Protection of the Educational Rights of Kids (PERK), a California-based child advocacy group, last week scored two important medical freedom victories for California schoolchildren and their parents.
One of those victories involved the Los Angeles Unified School District (LAUSD), the second-largest school district in the U.S., which on April 28 announced it is recommending and will vote on May 10 to delay the requirement for COVID-19 vaccines for students until July 1, 2023.
The announcement followed an April 25 ruling by Judge Mitchell L. Beckloff overruling the district’s motion to dismiss a lawsuit brought in by CHD-CA and PERK against LAUSD alleging the district lacked the legal authority to impose a COVID-19 vaccine requirement on its students aged 12 years and older.
Judge Beckloff also overruled the district’s motion to dismiss other claims, recognizing that CHD-CA and PERK had presented legitimate claims that could proceed in court.
On Jan. 14, LAUSD voted to delay implementation of the mandate and removal of students until the fall of 2022. CHD-CA will hold a rally on May 10, when the LAUSD board votes to delay implementation of the mandate until July 2023.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.
© 2022 Children’s Health Defense, Inc. This work is reproduced and distributed with the permission of Children’s Health Defense, Inc. Want to learn more from Children’s Health Defense? Sign up for free news and updates from Robert F. Kennedy, Jr. and the Children’s Health Defense. Your donation will help to support us in our efforts.

